INDICATIONS FOR PERMANENT PACING
The indications for permanent pacemakers can
be divided into three classifications (Table1)
and are listed in Table 2 (below) according to the most recent
indications published by a joint task force by the American
College of Cardiology and American Heart Association in 1998.
TABLE 1- Consensus for Appropriateness of
Pacer Implant Indication
Class I Conditions for which there is general
agreement that permanent pacemakers should be implanted.
Class IIa Conditions for which permanent pacemakers
are frequently used but there is divergence of opinion with
respect to the necessity of their insertion. Weight of evidence/opinion
is in favor of pace-maker use.
Class IIb Conditions for which permanent pacemakers
are frequently used but there is divergence of opinion with
respect to the necessity of their insertion. Weight of evidence/opinion
is in favor of pace-maker use.
Class III Conditions for which there is general
agreement that devices are unnecessary.
| |
Class I |
Class II |
Class
III |
Acquired AV block |
Third-degree AV block with: Bradycardia and symptoms
due to AV block
Requirement of drugs that result in symptomatic bradycardia
After catheter ablation of the AV junction or after postoperative
AV block not expected to resolve Neuromuscular diseases
with AV block
Escape rhythm <40 bpm or asystole >3 s in awake
symptom-free patients
Second-degree AV block. permanent or intermittent- with
symptomatic bradycardia |
Asymptomatic complete AV block with average awake ventricular
rate >40 bpm
Asymptomatic type 11 second-degree AV block (permanent
or intermittent)
Asymptomatic type I second-degree AV block at or below
the bundle of His (documented by electrophysiologic studies)
First degree AV block with symptoms suggestive of pacemaker
syndrome and documented alleviation of symptoms with temporary
pacing
Class IIb
Marked first-degree AV block in patients with congestive
heart failure
|
Asymptomatic first-degree AV block Asymptomatic type
I second-degree AV block above the level of the bun dle
of His
AV block expected to resolve |
|
After
myocardial infarction |
Persistent second- or third-degree AV block in the His-Purkinje
system or Transient advanced infranodal AV block and associated
BBB Symptomatic second- or third-degree AV block at any
level |
Class IIb
Persistent advanced AV block at the AV node level
|
Transient AV conduction disturbances without intraventricular
conduction defects or with isolated left an- terior fascicular
block
Acquired left anterior fascicular block Persistent first-degree
AV block in the presence of old or age-indeteminate BBB
|
|
Bifascicular or trifasicular block |
Intermittent
complete heart block associated with symptoms
Type II second-degree AV block |
Class IIa
Bifascicular or trifascicular block with syncope not proven
to be due to AV block but other causes of syncope not
identifiable
HV interval >100 ms or pacing-induced infra-His block
|
Fascicular block without AV block or symptoms
Fascicular block with first-degree AV block without symptoms
|
| Sinus node dysfunction |
Sinus node dysfunction with documented symptomatic bradycardia
(in some patients, this will occur as a result of long-term
essential drug therapy of a type and dose for which there
is no acceptable alternative)
Symptomatic chronotropic incompetence
|
Class IIa
Sinus node dysfunction. occurring spontaneously or as
a result of necessary drug therapy with heart rates <40
bpm without clear association between significant symptoms
and bradycardia
Class IIb
In minimally symptomatic patients, chronic heart rate
<30 bpm while awake |
Sinus
node dysfunction in asymptomatic patients, including those
in whom substantial sinus bradycardia is a consequence
of long-term drug treatment
Sinus node dysfunction in patients in whom symptoms suggestive
of bradycardia are clearly documented not to be associated
with a slow hear rate.
Sinus node dysfunction with symptomatic bradycardia due
to nonessential drug therapy |
|
Hypersensitive carotid sinus and neurocardiac syndromes
|
Recurrent syncope associated with clear, spontaneous events
provoked by carotid sinus stimulation: minimal carotid
sinus pressure induces asystole of >3 s duration in
the absence of any medication that depresses the sinus
node or AV conduction |
Class IIa
Recurrent syncope without clear, provocative events and
with a hypersensitive cardioinhibitory response
Class IIb
Syncope with associated bradycardia reproduced by head-up
tilt (with or without provocative maneuvers or isoproterenol
) |
A hyperactive cardioinhibitorv response to carotid sinus
stimulation in the absence of symptoms
Vague symptoms (dizziness or light-headedness) with a
hyperactive cardioinhibitory response to carotid sinus
stimulation
Recurrent syncope, light-headedness, dizziness in the
absence of a cardioinhibitory response |
Table
2
These recommendations serve as guidelines,
and there are other clinical factors that may affect the decision
to implant a pacer. Many indications for pacemaker implantation
are predicated by the presence of symptoms. However, many symptoms
such as fatigue or subtle symptoms of congestive heart failure
may be recognized only in retrospect, after placement of a permanent
pacemaker.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992
Pacing in Acquired Atrioventricular
Block
It is generally agreed that complete
heart block, permanent or intermittent, at any anatomic level
associated with symptoms such as dizziness, lightheadedness,
syncope, congestive heart failure, or confusion is an indication
for a permanent pacemaker. In the absence of symptoms, pacing
is indicated for patients with third-degree AV block, especially
with awake heart rates of less than 40 beats per minute or pauses
of longer than 3 s.
In the presence of bifascicular or trifascicular block, intermittent
third-degree or type II second-degree AV block usually indicates
the need for a permanent pacemaker. When patients with these
conduction patterns present with syncope, a pacemaker is usually
required. However, an electrophysiology study may be useful
to rule out other causes of syncope (e.g., ventricular tachycardia)
particularly if structural heart disease is present. Additionally,
during electrophysiology study, permanent pacing may be indicated
if there is a markedly prolonged HV interval (>100 ms) or
nonphysiologic pacing or drug-induced infranodal His block.
Second-degree AV block associated with
symptomatic bradycardia is an indication for pacing. In asymptomatic
pattients with second-degree AV block, type II, cardiac pacing
may be required if the level of block is infranodal because
the progression to complete heart block is common. Although
type I second degree AV block is usually located at the AV nodal
level,there are patients with bundle branch block or intraventricular
conduction delays in whom type I second degree AV block is located
at an infranodal level. These patients should be approached
similar to second-degree type II AV block, since the risk of
progression to complete heart block remains high. Lastly, with
2:1 AV block, the level of block may be difficult to determine.
In the presence of a bundle branch block or intraventricular
conduction delay and 2;1 Av block, the level of block is usually
infranodal and therefore may be an indication for pacing.
In asymptomatic and otherwise healthy
patients, the presence of intermittent second-degree, type I
AV maybe due to enhanced vagal tone. In asymptomatic elderly
patients with daytime type I second degree AV block and strutural
heart disease, however, there is some divergence of opinion
as to whetheher permanent pacing should or should not be considered.
Many patients may become symptomatic during clinical follow-up.
Due to the benign prognosis first-degree
AV block is not considered an indication for permanent pacing.
However, marked first-degree AV block (PR > 0.30 s), inappropriate1y
timed atrial systole that occurs after ventricular systole can
lead to symptoms similar to having retrograde ventriculoatrial
conduction. This may be of hemodynamic connsequence in some
patients. particularly with left ventricular systolic or diastolic
dysfunction. Additionally, because there is not an appropriately
timed ventricular systole occurring at the end of atrial sysole,
end diastolic mitral regurgitation develops, which may be of
clinical signifance in patients with left ventricular systolic
dysfunction. Therefore, dual chamber pacing may be indicated
in select patients with marked first dgree AV block in whom
hemodynamic improvement can be demonstrated by temporary pacing
to resynchronize the atrium and the ventricles.
Of note, patients with neuromuscular diseases
with AV block should be considered for DDDpacing, since progression
of conduction system disease is not uncommon.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992
Pacing in Congenital Atrioventricular
Block
Congenital heart block is usually due
to AV nodal block. Patients tend to be asymptomatic and typically
have narrow QRS complex rhythms. However, congenital AV block
is associated with serious and possible fatal complications,
including syncope and sudden death. In one study, a mean daytime
heart rate less than 50 beats per minute was associated with
sudden death or need for pacemaker. Exercise testing is useful
to assess response at rest and exercise. Other indicators of
poor outcome include prolonged QT interval (corrected for heart
rate), cardiomegaly. atrial enlargement. decreased left ventricular
systolic function, mean ventricular rates lower than median
for age, periods of junctional exit block, and mitral regurgitattion.
Therefore, cardiac pacing is indicated
in all symptomatic patients with congenital AV block. Furthermore,
cardiac pacing is now recommended even for symptom-free adults.
In the largest series published to date, there was reported
a 5 percent mortality risk in adults older than 15 years with
congenital AV block in the absence of heart disease. Eight of
102 patients whose cases were followed for 7 to 30 years had
fatal Stokes-Adams attacks. Syncope, mitral regurgitation, and/or
heart failure occurred in 30 percent of this cohort.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992
Pacing in Sinus Nodal Dysfunction
Sinus nodal dysfunction has become the most
common indication for pacing in the United States. Pacing therapy
has been demonstrated to be superior to medical therapy with
theophylline for patients with sinus nodal dysfunction. The
guidelines (Table
1 and Table 2 above) stress the importance of correlating
symptoms with bradyarrhythmias. Often, it is difficult to correlate
ECG findings with symptoms. Furthermore, symptoms may be nebulous.
For instance, the presence of fatigue and dyspnea may be due
to a bradyarrhythmia but may also be duee to lack of conditioning
or other cardiac dysfunction.
The presence of the tachycardia/bradycardia syndrome is especially
common in patients with paroxsymal atrial arrhythmias (Fig.
16 fourth illustration). The bradyarrhythmia often occurs
at the termination of tachycardia and can lead to pauses of
several seconds.
Drugs used to suppress tachyarrhythmias may lead to symptomatic
bradycardia, in which case a bradycardia pacemaker would be
required.
Patients with asymptomatic bradyarrhythmias
should be evaluated carefully prior to placing a pacemaker.
In general, an absolute heart rate of less than 30 beats per
minute is an indication for pacemaker placement, even in the
absence of symptoms. An exercise test can demonstrate intact
sinus nodal function in patients with otherwise asymptomatic
bradyarrhythmias who do not require pacing therapy. Atheletes
commonly have physiologic bradycardia. even with heart rates
of less than 40 beatd per minute, due to enhanced vagal tone.
Finally, it should be noted that sleep apnea may cause asymptomatic,
nocturnal bradyarrhythmias, in which case pacing therapy is
not indicated.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Pacing in Carotid Sinus Syndrome
The diagnosis for carotid sinus syndrome
(CSS) is typically made by demonstrating asystolic pauses of
longer than 3 s with carotid sinus massage or a vasodepressor
response of greater than 50 mmHg associated with clear symptoms
provoked by carotid sinus stimulation, such as wearing a tight
shirt or turning one's head. Vague symptoms such as dizziness
associated with a hyperactive cardioinhibitory response to carotid
sinus stimulation do not represent an indication for permanent
pacing.
Improvement of symptoms and suppression of syncope have been
demonstrated by treating patients with cardiac pacing, particularly
dual-chamber pacing. Single-chamber atrial pacing is contraindicated
because of the increased risk of transient AV block. Some studies
suggest that hemodynamic evaluation of patients may enable them
to be stratified into groups among whom VVL pacing would be
sufficient. However, DDD pacing is probably better in most patients
with CSS, because of the presence of vasodepressor and cardioinhibitory
reflexes.
Cardiac Pacing in Neurocardiogenic
Syncope
The role of pacing for neurocardiogenic
syncope is controversial. Because these are younger patients who
generally respond to medication, pacing is not required in most
patients. Cardiac pacing has been shown to prevent the bradycardia
and AV block associated with neurocardiogenic syncope, but patients
still typically experience hypotension, vasodilatation, and other
associated symptoms. The Vasovagal Pacemaker Study demonstrated
a role for pacing in patients with vasovagal syncope refractory
to standard medical therapy. Therefore, patients with refractory
neurocardiogenic syncope may benefit from pacing, especially if
they have a predominant cardioinhibitory component.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Pacing in Hypertrophic Cardiomyopathy
In patients with hypertrophic cardiomyopathy
(HCM) and left ventricular outflow tract (LVOT) gradients, DDD
pacing with a programmed short AV interval has been proposed
as therapy to reduce LVOT gradient and improve symptoms. This
concept is based on early studies where it was shown that DDD
pacing with short AV interval decreased the LVOT gradient by
a mean of 35 mmHg, and there was improvement of symptoms associated
with HCM. An observational study involving 84 patients whose
cases were followed for a mean of 2.3 years showed improvement
of symptoms in nearly all patients, and there was reduction
in the left ventricular wall thickness by more than 4 mm in
a subgroup of patients. However, 15 percent of the patients
required AV junction ablation to allow ventricular preexcitation
by the pacer.
The mechanism by which DDD pacing reduces
LVOT gradient remains controversial. With ventricular pacing
at short AV interval, the right ventricular apex is preexcited
by the pacemaker, causing alteration of the left ventricular
activation sequence and paradoxical septal motion. This causes
the septum to move away from the posterior left ventricular
wall in early systole, thereby widening the LVOT during systole.
It is also possible that ventricular pacing alters myocardial
perfusion, decreases mitral valve systolic anterior motion,
and/or decreases inotropy, which may also contribute to the
effects of pacing in this disorder.
Therefore, if DDD pacing is used as terapy for obstructive hypertrophic
cardiomyopathy, placement of pacing lead and programming of
the AV interval are crucial for a beneficial effect. The AV
interval should be programmed to the interval that still allows
for left ventricular preexcitation,which would decrease but
not eliminate the deleterious pacing with very short AV intervals.
Echocardiography may help select the optimal pacing AV interval.
The long-term clinical effectiveness of DDD pacing in patients
with obstructive HCM remains controversial. Some recent and
randomized studies cast some doubt as to the clinical effectiveness
of pacing for objectively improving functional capacity, quality
of life, and LVOT gradient. One small randomized study failed
to demonstrate improvement in exercise response to DDD pacing.
Another study in patients with obstructive HCM showed that when
patients were randomized to backup AAI pacing versus DDD pacing
with short AV interval, there was no difference in subjective
improvement. LVOT gradient was reduced by 40 percent in 57 percent
of patients and remained unchanged in the other 43 percent of
patients. Only 12 percent of patients (all older than age 65)
showed improvement in functional capacity after 12 months in
the study. Therefore, based on this randomized double-blind
study,pacing could not be routinely recommended for drug-refractory
patients with obstructive HCM but, rather, may be considered
for select patients with medically refractory obstructive HCM
as an alternative to surgical myectomy.
Patients with hypertensive cardiac hypertrophy with cavity obliteration
may also show clinical improvement with DDD pacing. In contrast
to obstructive HCM, patients with nonobstructive symptomatic
HCM experience limited symptomatic improvement and no objective
evidence of hemodynamic benefit with DDD pacing and short AV
interval.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Pacing in Dilated Cardiomyopathy
and Congestive Heart Failure
Initial reports suggested that patients with
congestive heart failure and dilated cardiomyopathy may benefit
from dual chamber pacing by altering and optimizing timing of
left atrial to left ventricular activation or improving left
ventricular contractile function. There was initial enthusiasm
that pacing with a short AV interval may improve hemodynamic
function and that the patients with first-degree AV block derived
the most benefit. An acute hemodynamic study demonstrated that
pacing with a short AV interval could eliminate presystolic
mitral regurgitation in patients with first-degree AV block,
restore normal AV relationships, and improve hemodynamic function.
Subsequent studies showed that standard DDD pacing does not
improve hemodynamic function in patients with physiologic PR
intervals. On this basis, DDD pacing is possibly indicated in
patients with dilated cardiomyopathy or marked first-degree
AV block and where acute hemodynamic studies demonstrate improvement
by dual chamber pacing.
Whereas pacing the ventricles with short AV
interval may have limited benefit, the ability to pace the ventricles
in a more synchronous manner to improve mechanical efficiency
has also been studied. Ventricular pacing typically is achieved
by pacing through a lead placed in the right ventricular apex,
which may not produce the most efficient ventricular mechanical
function. Pacing through the His-Purkinje system in theory may
provide more physiologic ventricular activation patterns but
is currently not readily available. Pacing from the right ventricular
septum or outflow track may enable earlier left ventricular
activation and, hence, more simultaneous contraction. However
the results of hemodynamic improvement using right ventricular
outflow tract pacing has shown a trend for improvement in some
studies,whereas other studies show no benefit at all compared
ventricular apical (RVA) pacing. It has been proposed that left
ventricular or biventricular pacing may optimize hemodynamic
function in patients with dilated congestive cardiomyopathY,
particularly those patients with intraventricular conduction
delay. Left ventricular pacing can be accomplished by either
an epicardial lead or a transvenous lead through the coronary
sinus venous system. An acute hemodynamic study was performed
on patients with severe heart failure, intraventricular conduction
delay (usually bundle branch block), and increased capillary
wedge. These patients had measurement of hemodynamic parameters
during either right ventricular pacing or biventricular pacing,
which was compared with AAI pacing (control values). These results
showed improvement of cardiac index and decrease in capillary
wedge pressure with either right ventricular pacing or biventricular
pacing, which was compared with AAI (control values). These
results showed improvement of cardiac index and decrease in
capillary wedge with either right ventricular pacing or biventricula
pacing compared with AAI pacing. Furthermore, biventricular
pacing showed more hemodynamic benefit compared with right ventricular
pacing. Another study on patients with congestive heart failure
and wide duration showed that epicardial left ventricular pacing,
with or without concurrent right ventricular pacing, mproved
hemodynamic function at optimized AV intervals compared with
controls values. Therefore, left ventricular pacing may evolve
as a therapeutic pacing technique in patients with congestive
dilated cardiomyopathy and intraventricular conduction delay.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Another study has recently shown that biventricular
pacing may improve maximal and submaximal exercise capacity
in patients with advanced heart failure and intraventricular
conduction delay.
Varma,C.,MD,and others,JACCC,Vol.41,No.4,2003,PP582-588.
It has been recently reported that functional
mitral regurgitation is reduced by cardiac resynchronizatio
therapy (implantation of a biventricular pacing device with
a right ventricular apical lead and a LV pacing electrode implanted
through the coronary sinus and positioned in an LV epicardial
vein) in patients with heart failure (HF) and left bundle branch
block (LBBB). This effect is directly related to the increased
closing force (LV+dP/dt max. The results support the hypothesis
that an increase in transmitral pressure gradient(TMP),mediated
by a rise in LV+dp/dtmax due to more coordinated LV contraction,
may facilitate effective mitral valve closure.
Breithhardt,MD,and others,Acute Effects of
Cardiac Resynchronization Therapy on Functional Mitral Rgurgitation
in Advanced Systolic Heart Failure,JACCC,Vol.41,No.5,2003,PP
765-770.
PACEMAKER HARDWARE
Implant and Explant
Nearly all pacemakers are implanted through
a transvenous approach by either cardiologists or surgeons.
The choice of using an operating room or a catheterization Iaboratory
for the implant procedure probably plays little role in procedural-related
complications but a cardiac catheterization laboratory involves
lower hospital costs.
A full description of the surgical procedure
has been reviewed elsewhere. Venous access for lead placement
is through a subclavian venipuncture or a cephalic vein cutdown.
The use of subclavian venipuncture is technically easier, and
this vein can almost always accommodate two leads. With the
subclavian venipuncture, there exists the risk of subclavian
artery puncture, pneumothorax, or air embolus. Furthermore,
pacing leads placed medially incur an additional risk of being
"crushed" by the clavicle and first rib leading to lead insulation
breaks or fractures (Fig.
16b). Lateral puncture of the subclavian or axillary vein
using intravenous contrast may allow for safe lateral subclavianl
venous puncture. A cephalic vein cutdown may also avoid some
of the risk associated with subclavian vein puncture; however,
this vein is not always accessible and cannot always accommodate
two pacing leads.
Explanations of pacemaker generators are routinely
performed during pacemaker generator changes. However, removal
of pacemaker leads can be difficult due to fibrosis between
chronically implanted leads and surrounding cardiac, valvular,
and vascular structures. Traditional methods for extraction
of chronically implanted leads involve specialized extraction
sheaths that are glided over implanted leads to tear and peel
away the encapsulating tissue. Recently, a technique using ultraviolet
excimer laser light has been introduced to facilitate lead extraction
by allowing advancement of sheaths over pacer leads without
excessive mechanical tearing of fibrotic tissues. Compared with
mechanical extraction, laser-assisted extraction demonstrated
a greater success rate in lead removal (94 percent versus 64
percent) and less time to remove leads, with no difference in
complications.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Hardware
The pacemaker system consists of a pulse generator
and the pacing lead(s). Pacemaker system selection should be
primarily based on the medical and surgical requirements of
the patient. It is unusual that one pacemaker system would be
most optimal and cost effective for all patients. An algorithm
for choosing a pacemaker system and pacing mode is presented
in Fig.
16c. Pacemaker leads can be unipolar or bipolar (Fig.
16d and Fig.
16e). Unipolar leads use a distal electrode in the catheter
as the cathode and the shell of the pacemaker generator as the
anode. Therefore, the myocardium and adjacent tissue complete
the circuit. A bipolar lead consist of two separate conductors
and electrodes within the lead. Since the electrodes for sensing
in a bipolar lead are much closer together, bipolar signals
are sharper with less extraneous noise (Fig.
16f).
Unipolar leads are simpler to design, smaller
in diameter and, because of their simplicity, probably less
likely to fail. Because of their small size, it is easier to
pass two unipolar leads through a cephalic venous approach.
However there are several disadvantages to unipolar lead systems.
Because the unipolar lead uses body tissue to complete the circuit,
there is the possibility of causing muscle stimulation. Most
pacemakers avoid this by placing the stimulating surface of
anterior such that it interfaces with subcutaneous tissue and
not the pectoralis muscle. Unipolar sensing is fa to pick up
extracardiac signals, including myopotentials (Fig.
16f), far-field sensing of remote cardiac potentials and
electromagnetic interference. Finally, unipolar pacing is generally
contraindicated in patients with a concomitant implantable defibrillator.
Therefore, most leads implanted today are bipolar.
Leads are attached to the heart by active
or passive fixation. Active fixation involves the use of some
type of exposed or retractable screw within the lead system
that fixes the lead to the heart (Fig.
16e). Passive fixation involves the use of tines, which
are short protuberances that extend proximal to the distal electrode
and interact with myocardial tissue to hold the lead in place.
Active fixation leads are used more in the atrrium and allow
fixation of the leads almost anywhere within the right atrium
or ventricle. The use of either type of lead probably has little
effect on complication rate or lead dislodgment rate when used
by experienced operators.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Lead Placement and Acute Threshold Testing
Atrial and ventricular leads are placed into
the appropriate chambers after ensuring adequate pacing and
sensing thresholds.

Table 3
The basic premise in obtaining acute pacing
and sensing thresholds during implant is that these thresholds
may degenerate over time, and adequate safety margins need to
be obtained to ensure safe long-term pacing and sensing. Furthermore,
one should be aware of the type of unit implanted, its capabilities
for pacing outputs, programmed sensitivities,and pacing modality
(bipolar versus unipolar). The indication for pacing may also
affect decisions about acceptable pacing thresholds, because
of the inverse relationship between current drain and battery
life. In patients who only require occasional backup pacing,
higher pacing thresholds may be acceptable. Therefore, pacing
thresholds should be optimized at the time of implant as influenced
by the patient's pacing requirements and capabilities of the
pacemaker. For sensing functions, ventricular electrograms measure
at least 5 mV at frequently measure in excess of 10 to 20 mV.
Ventricular sensitivity is generally programmed between 2 to
3 mV so that adequate safety margin exists for intrinsic ventricular
depolarizatlion without the risk of oversensing T waves or other
artifacts. Atrial electrograms are lower in amplitude than ventricularelectrograms;
however, a minimum atrial electrogram to 2 mV should be obtained.
In unipolar systems, a largei electrogram is important because
of the increased risk ol sensing myopotentials or other artifactual
signals if the sensitivity is programmed to less than 1 mV.
In patients paroxysmal atrial fibrillation or flutter, the atrial
during tachycardia might be smaller than during sinw Conversely,
in patients with marked sinus bradycardia it is expected that
there will be nearly 100 percent atriai atnal sensing thresholds
may not be as important. Finalbi minimum programmed sensitivity
available by the pacer I 0.5 mV) may influence acceptable sensing
thresholds al Many factors may affect atrial or ventricular
pacing sensing thresholds. There is variation to these threshold
pending on the autonomic tone or the electrolyte status is an
expected rise in acute thresholds within 1 to following implant
due to acute inflammation, which be more exaggerated with active
fixation lead syste] drugs, particularly antiarrhythmic medications,
may ing thresholds. The presence of new myocardia around the
leads would be expected to lead to dete pacing and/or sensing
thresholds. Leads that are steroid eluting generally limit the
acute rise in pacing threshold. Long-term thresholds appear
to stabilize sometime after 3 to 6 months.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
PACEMAKER FOLLOW-UP
The goal for pacemaker follow-up should be
to perform a systematic evaluation of the pacemaker as it relates
to and functions with the patient and his or her individual
needs. These goals are outlined in Table
4. Complete guidelines for pacemaker follow-up have been
described.
In the first several months after pacer implant, several evaluations
of pacer function may be required in order to optimize pacing
outputs, rate responsiveness, and other features. There is a
stable period of pacer function starting 6 to 12 month following
implant until the expected time for battery depletion. Therefore,
direct evaluations of pacer function may be performed once or
twice per year during this time, depending on whether the patient
is pacer dependent and depending on the pacer type and whether
any of the pacer components are under any advisory warnings.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Transtetephonic Monitoring
Technology is available for simple devices
used by patients to transmit their ECG by telephone to a receiving
station so that their ECG rhythm may be analyzed to detect normal
or abnormal pacemaker function. In this way, a spontaneous pacing
rhythm can be assessed for normal or abnornal pacing function.
More importantly, by applying a magnet to the pacemaker and
observing the magnet rate during the transtelephonic monitoring
(TTM), the battery status can be assessed During TTM, changes
in pacing rate or loss of output could always be detected. Ventricular
oversensing or atrial pace/sense problems can sometimes be detected
during TTM. Follow-up using TTM should be used to supplement
and replace direct evaluation of pacer function. The frequency
of follow-up should be individualized according to the type
of pacemaker, whether the patient is pacemaker dependent, age
of puIse generator and expected longevity, presence of any pacemaker
component under advisory or warning, and patient clinical factors.
As depletion of pacer battery occurs, TTM may used as often
as every month to appropriately determine timing for pacer replacement.
Components for Direct Evaluation of
Pacemaker Systems
CHECKING PACING THRESHOLDS AND PROGRAMMING
PACING OUTPUTS
Pacemakers should always be programmed for
maximal safety particularly in patients who are pacemaker dependent.
To understand how to program pacemakers safely and efficiently,
basic principles are reviewed.
Current Drain
Ultimately, the longevity of the battery
will be function of the current drain versus battery capacity.
There is nominal current drain for operating pacemaker circuitry,
which varies according to the pacer type; however, most current
drain results from pacing output.
The current delivered per pacing pulse is a function of the
voltagedivided by the lead impedance (I=V/R)-Ohm's law. Therefore,
it is desirable to be able to implant leads with low pacing
voltage thresholds. Additionally, leadsdesigned to have high
impedance appear to decrease long term current drain.
Strength-Duration Curve
The strength-duration curve (Fig.
16g) relates voltage and pulse width. This curve is dynamic
during the first 2 to 3 months following implant. With an acute
rise in threshold, the curve is expected to shift upward two
to four times and then subsequently shift back downward at a
level greater than the initially obtained values. At pulse widths
less than 0.2 ms, the curve is steep; at pulse widths exceeding
1.0 ms, the curve is flat. With this kind of relationship, programming
pulse widths greater than 1 ms generally does not add safety
margin to the pacing output but does substantially increase
battery current drain. Similarly, programming pacing pulse widths
less than 0.2 to 0.3 ms may not allow sufficient safety margin
at even high voltage amplitudes.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .
Total Energy Expenditure of the Pacemaker
This is defined as energy = (voltage)2 multiplied
by pulse width divided by impedance. According to this relationship,
the energy expenditure has an exponential relationship to voltage
output but has a linear relationship to pulse width. Therefore,
it is preferable to reduce voltage output rather than pulse
width to conserve battery life.
Calculating Pacing Threshold
At implant, it is standard to fix the pulse
width at 0.5 ms and reduce the voltage until the lowest voltage
that maintains consistent pacing-which is the pacing threshold
(Fig.
16h). One can fix the pulse width at any value, however
(usually between 0.3 and 1.0 ms), and calculate a voltage threshold.
Similarly, one can fix the voltage at a certain value and reduce
the pulse width to the lowest value that maintains consistent
pacing, which would also define the pacing threshold. Either
method is acceptable to define a pacing threshold.
Safety Margin
The safety margin for pacing outputs can be
calculated by multiples of either the pulse width or the voltage
threshold. For example, if the voltage threshold at 0.5 ms is
1.5 V, then a pacing output of o.5 ms and 3.0 V would yield
an energy safety margin of fourfold, given the relationship
between energy and voltage. Similar, if pulse-width threshold
at 3.0 V. is 0.15 ms, then a pacing output of 3.0 V and 0.6
ms would provide an energy safety margin of fourfold.
Acute Pacing Outputs
Because the extent of the acute rise in pacing
thresholds may be difficult to predict, it is better to program
high pacing outputs at implant and during the first 6 to 24
weeks after implant. A greater safety margin may be desired
in patients who are pacemaker dependent. Typically, greater
safety margins are also desired in ventricular leads rather
than atrial leads. Steroid-eluting leads generally result in
blunting of the acute rise in threshold, which may allow for
lower pacing outputs early after implant.
Chronic Pacing Outputs
In the time frame of 2 to 6 months, the pacing
thresholds stabilize. Therefore, chronic pacing outputs may
be programmed.

Table 5
Almost all pacing batteries consist of lithium-iodide
systems, which generate 2.8 V. It is most efficient to pace
at the voltage of the battery (2.5 to 2.8 V). Therefore, longevity
of pacemakers can be improved if pacing outputs are reduced
to 2.5 V with pulse widths programmed 2 to 4 times pulse-width
thresholds. Finally, some newer pacemakers have the ability
to confirm capture on a beat-by-beat basis. Using algorithms
to automatically check pacing capture thresholds, these pacers
adjust pacing voltages just above the pacing threshold in order
to reduce curent drain and prolong battery longevity.
OTHER FEATURES
Sensing
Sensing of atrial and ventricular intracardiac
electrograms can be evaluated by different algorithms. To test
atrial sensing, the pacemaker needs to be programmed temporarily
at a programmed atrial rate less than the intrinsic sinus rate.
To test ventricular sensing, the pacer can be temporarilly regrammed
to the VVI mode if the programmed rate is less than the intrinsic
heart rate. Alternatively, with intact AV conduction, the delay
can be increased to allow AV conduction and thereby allow for
ventricular sensing in the DDD mode. Increasing the programmed
sensitivity until the intrinsic P or R wave is no longer sensed
(Fig.
16i) is another method to test sensing threshold. Telemetry
of atrial or ventricular electrograms allows for direct measurement
of the amplitude (Fig.
16f). Lastly, some pacemakers have algorithms whereby the
pacemaker automatically measures atrial and ventricular electrograms.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992
Lead Function
Lead function is assessed by pacing and sensing
function and by measuring impedance. Although there is a wide
variability of normal lead impedances, chronic lead impedances
should not widely vary between outpatient follow-up visits.
A fractured lead exhibits a markedly elevated lead impedance.
Insulation breaks manifest by reduced lead impedances. Lead
fractures or insulation breaks often are intermittent problems.
Therefore, normal lead impedances and pacing and sensing thresholds
do not rule out these problems. The leads can be stressed by
having the patient change position and do various provocative
arm movements to facilitate diagnosis of lead-related problems
that are not otherwise observed.
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992
Battery function
Almost all pacemakers use lithium-iodide batteries,
which have an initial battery voltage of 2.8 V. Battery voltages
can be directly measured and, at a certain level (elective replacement
index, ERI), the pacemaker unit requires elective generator
change. At a lower voltage (end of life, EOL), there is potential
loss of pacemaker function; therefore immediate generator change
is mandated.
Battery function can also be assessed without formal interrogation.
Many pacemakers reset to a VVI mode at a preset pacing rate,
or the pacing rate decreases to less than the programmed lower
rate of the pacermaker when battery reaches the ERI or EOL stage.
Additionally, the magnet mode causes asynchronous pacing at
a preset magnet rate for particular pacemaker model. This magnet
rate varies according to whether the battery status is adequate
or not.
Rate Responsiveness
Rate-responsive pacemakers require periodic
adjustments of the rate-responsive features to optimize clinical
responsiveness. The programmable variables include a rate-responsive
upper pacing rate, which may be a separate programmable variable
than the upper tracking rate. A rate-response slope may be programmed
to determine the pacing rate at a certain activity level. Some
pacemakers store data with respect to the use of rate responsiveness
over a certain period. Otherwise, one can simply have the patient
walk briskly for 2 to 3 mm and assess the heart rate to determine
whether it is appropriate given the patient's age and clinical
status. Some pacemakers offer algorithms whereby the physician
chooses the appropriate heart rate for "brisk walking,"
and the pacemaker automatically calculates the optimal rate-responsive
programming.
Pacer Diagnostic Function
Modern pacemakers have increased memory capabilities
to store diagnostic information. The basic diagnostic feature
displays counts or percentages of pacing versus sensing in the
atrial and ventricular chambers. If a patient has complete heart
block but has intact sinus nodal function, it would be expected
that there be 100 percent ventricular pacing with predominant
atrial sensing. The breakdown of pacing and sensing in each
chamber can be stratified according to the heart rate that can
give the clinician some clues as to the presence of chronotropic
incompetence or appropriateness of rate responsiveness.
With respect to arrhythmia monitoring, the presence and quantity
of premature ventricular and atnal complexes are presented.
For patients with mode-switching pacemakers, the number of mode
switches probably represents a marker for the number of atrial
arrhythmias. However, these data do not provide information
with respect to duration and timing of these atrial arrhythmias.
One study showed that most of these atrial arrhythmias are very
brief, lasting only a few seconds in many cases More information
about the occurrence, timing, and duration of arrhythmias, including
stored intracardiac electrograms, is available in some pacers.
This type of information may facilitate diagnosis of arrhythmias
without the need for ancillary testing (Fig.
16j). Furthermore, when patients complain of symptoms such
as palpitations, these diagnostic features may enablediagnosis
of, or rule out, atrial or ventricular tachyarrhythmias.
Chest Radiograph (Posteroanterior and Lateral)
A standard chest x-ray is recommended as
part of the predischarge evaluation to ensure appropriate placement
of leads, rule out lead migration, and serve as a baseline.
PACEMAKER FUNCTION AND MODES
Magnet Mode
Virtually all pacemakers pace in an asynchronous
mode when they come into contact with a magnetic field. The
response to a magnet varies according to manufacturer, pacemaker
model, and sometimes even the mode in which a pacer is programmed.
Single-chamber pacers respond to magnets by asynchronous pacing
at either the programmed rate or a special magnet rate (Fig.
16k). This allows a simple noninvasive method to assess
pacing at the bedside, office, or by TTM. In patients who are
pacemaker dependent and experiencing oversensing thereby inhibiting
pacemaker output, a magnet is a convenient short-term method
to ensure pacing. Furthermore, pacemakers usually have one magnet
rate for a battery that is intact and another one for a battery
that is at ERI or at EOL. If these rates are known, applying
a magnet to a pacemaker is an easy noninvasive method to assess
battery status.
VVI Mode
In the VVI mode, a pacemaker operates as shown in Fig.
16l The lower rate is converted to an interval (milliseconds).
After a paced or sensed ventricular event, a programmable refractory
period prevents inappropriate sensing of T waves. After the pacemaker
ventricular refractory period, there is an interval extending
to the escape interval during which time the pacemaker senses
a ventricular event, if one occurs before the end of the interval;
otherwise, there is ventricular pacing output.
Hysteresis is a programmable function in which the ventricular
escape interval is longer after a sensed ventricular event than
after a paced ventricular event. This feature can be used in patients
with sinus rhythm so that VVI pacing would not initiate until
the sinus rate drops below the hysteresis rate, which is lower
than the pacemaker rate (Fig. 16m
).
AAI Pacing
AAIR is an excellent mode of pacing in
patients with sinus node dysfunction and normal AV nodal and
His-Purkinje function.The timing sequences are the same for
AAI as for VVI pacing. Atrial sensitivities are programmed at
lower values (increased sensitivity) to sense intrinsic P waves
safely. This frequently leads to oversensing of far-field ventricular
electrograms, which can be avoided by programming a longer refractory
period.
Patients with sinus nodal dysfunction may develop AV block,
which may be a source of concern when using AAI pacing. However,
with careful selection of patients, including normal PR intervals,
absence of bundle branch block, and AV Wenckebach occurring
at atrial pacing rates of more than 120 beats per minute, the
risk of development of second- or third-degree AV block is less
than 0.6 percent per year.
DDD Pacing
DDD pacing is the most common pacing
mode for dual-chamber pacemakers. The timing sequences for DDD
pacing are described in
Fig. 16n. This mode is used for patients with AV node and/or
sinus node dysfunction.
DDD PACING IN PATIENTS WITH SINUS
NODE DYSFUNCTION
Patients with sinus node dysfunction
may have intermittent or chronic sinus bradycardia requiring
intermittent or continuous atrial pacing. If patients have intact
AV conduction, the pace-maker functions as an AAI pacer. Due
to medications that slow AV conduction and/or intrinsic AV nodal
or His-Purkinje disease, however, patients with DDD pacemakers
frequently demonstrate fused ventricular complexes originating
from ventricular stimulation and through the AV conduction system.
The degree of fusion of the ventricular complex between pacing
from a right ventricular lead and conduction down the AV nodal-His-Purkinje
system depends in large part on the difference between the programmed
AV interval and the intrinsic AV conduction time.
For ventricular output to be inhibited in patients with DDD
pacemakers, the pacemaker AV interval must be longer than the
conduction time between the sensed or paced atrial complex to
the right ventricular lead. A very long AV interval (more than
0.25 s) may decrease the benefit of AV synchrony when AV pacing
does occur. It is not uncommon that pacemakers sense the ventricular
electrogram late during ventricular depolarization especially
with right ventricular conduction delay or right bundle branch
block. Pacemaker pseudofusion occurs when there is ventricular
pacing within the QRS complex (Fig.
16o).
PATIENTS WITH ATRIO VENTRICULAR BLOCK
AND NORMAL SINUS NODE FUNCTION
In the DDD mode, if the lower rate of
the pacer is programmed at a sufficiently low value to permit
atrial tracking, the pacemaker stimulates the ventricle synchronous
with intrinsic P waves. If a patient does not require atrial
pacing, it may be reasonable to implant a dual-chamber pacer
with a single tripolar or quadripolar lead that allows atrial
sensing and ventricular pacing and sensing (Fig.
16p). These VDD pacing systems allow for ease of implant
and for bipolar atrial sensing. Atrial sensing may not be as
reliable compared to a fixed atrial lead, which may lead to
occasional atrial undersensing.80'81 In a recent prospective
comparison between single-lead VDD systems to DDD leads, however,
there were lower P-wave amplitudes in the group with VDD systems,
but no significant clinical differences with respect to atrial
undersensing.
DDD versus VVI Pacing
Multiple retrospective and observational
studies and a few 'rospective studies demonstrate hemodynamic,
clinical, and quality-of-life benefits of dual-chamber or atrial-based
pacing versus ventricular pacing. Therefore, it appears prudent
to implant DDD pacers in most patients with intact atrial function
but not all patients.
In patients with congestive heart failure due to left ventncular
systolic dysfunction, the dependence of cardiac output to AV
synchrony appears to decrease secondarily to the already increased
left ventricular filling pressures. Patients with fixed stroke
volume (i.e., left ventricular systolic dysfunction) may depend
almost exclusively on heart rate for cardiac output and, therefore,
may have limited benefit from AV synchrony. However, any improvement
in cardiac output with restoration of AV synchrony may be clinically
significant. Additionally, clinical conditions such as left
ventricular hypertrophy or diastolic dysfunction generally are
dependent on adequate preload to maintain cardiac output. Restoration
of AV synchrony appears to be particularly significant for these
patients.
Patients with Sick Sinus Syndrome
In patients with sick sinus syndrome,
dual-chamber pacing has been shown to be superior to VVI pacing
Many studies have demonstrated that atrial-based pacing (DDD)
is associated with decreased clinical events, including atrial
fibrillation, congestive heart failure, stroke, and death, mainly
but not exclusively in patients with sick sinus syndrome. Several
mechanisms by which atrial-based pacing is beneficial in patients
with sick sinus syndrome may not apply to the subset of patients
with ciegree or complete AV blocK. VVI pacing in patients with
retrograde VA conduction causes atrial contractions against
closed AV valves, leading to atrial distension and transient
increases in pulmonary capillary wedge and jugular venous pressures.
Increased atrial distension may predispose individuals to atrial
fibrillation. This is apt to be more evident in the patients
with sick sinus syndrome who already have paroxysmal atrial
fibrillation or are at risk for such arrhythmias. Sympathetic
activity is elevated during VVI versus dual-chamber pacing,
which contributes to increased morbidity and possible mortalityY9
Even in the absence of retrograde conduction, VVI pacing with
VA dissociation leads to atrial systoles throughout the cardiac
cycle, which can also lead to a similar deleterious effect on
atrial size and function. Therefore, dual-chamber pacing appears
to reduce the incidence of atrial fibrillation and embolic complications.
Andersen and colleagues published short- and long-term reports
on a randomized study comparing single- and dual-chamber pacing
in patients with sick sinus syndrome. In their long-term study,
they reported a reduction of embolic events, atrial fibrillation,
and mortality with use of atnal-based pacing. Additionally,
they found progressive benefit from atnal pacing compared with
ventricular pacing, which resulted in overall improvement of
survival based on total mortality and death from cardiovascular
causes. Additionally, many studies have shown that maintenance
of AV synchrony improves quality of life particularly at rest.
In fact, many patients who have VVI pacers may not recognize
the extent of their symptoms until they have an upgrade to a
DDD system.
Atrioventricular Block
In patients with AV block, the advantage
of dual-chamber pacing has been demonstrated by some authors
but not by others. In a retrospective study from the Mayo Clinic
on an elderly population, long-term survival was not affected
by the mode of pacing. Lamas et al published a series on 407
elderly patients (older than age 65) who were randomized to
have a dual-chamber pacer programmed to either VVI~RJ or DDD~R]
modes. These authors concluded that the main quality-of-life
benefits associated with DDDR pacing were noted in the group
of patients with sick sinus syndrome, and there were no quality-of-life
benefits noted in the patients with pacers implanted for AV
block.
Therefore, there now appear to be adequate data supporting the
use of atrial-based pacing (AAI, DDI, and DDD) in patients with
sick sinus syndrome. The benefit of dual-chamber versus ventricular
pacing in patients with advanced or complete AV block appears
to be controversial. In patients with intact sinus node function
and AV block, however, it is prudent to at least implant a single-lead
VDD system, if not a complete dual-chamber pacing system, to
restore AV synchrony in order to restore physiologic pacing.
PACING TIMING INTERVALS AND UPPER
RATE BEHAVIOR
Atrioventricular Interval
The Av interval is divided into three
zones.The first 20 t0 40ms of this interval is the atrial blanking
period.The ventricular channel is blanked during this period
to prevent inappropriate sensing of atrial output (crosstalk).
Crosstalk is a greater problem in unipolar than in bipolar systems.
The next part of the AV interval occurs from the end of the
blanking period to approximately 100 to 120 ms after the atrial
pacing output. If a ventricular sensed event occurred at this
point, it would be nonphysiologic because of the short elapsed
AV interval. The pacemaker responds with a ventricular output
at a short AV interval (100 to 120 ms), which is a safety feature
(ventricular safety pacing (Fig.
16q).Ventrcular safety pacing is a feature that ensures
ventricular pacing in case the sensed event was not a ventricular
depolarization;instead, pacing occurs at a short interval so
that the pacing output falls before the T wave.
Finally, if there is a sensed event in the
latter part of AV interval, the pacemaker response is to inhibit
ventricular pacing output.
Upper Rate Behavior
The total atrial refractory period (TARP)
consists of the AV interval and the postventricular atrial refractory
period (PVARP). The TARP is a programmable value that can be
calculated in milliseconds. Ventricular tracking of atnal events
cannot exceed a frequency shorter than the TARP. By dividing
60,000 by the TARP, a rate can be calculated that is the upper
rate at which a pacemaker can track atrial events at a 1:1 ratio.
At atrial rates exceeding this value, every other atrial event
will fall within the pacemaker refractory period (PVARP) and
there will be 2:1 pacemaker AV block. Therefore, the rate corresponding
to the TARP corresponds to the pacemaker 2:1 rate.
The upper tracking rate is a separate programmable value. The
upper tracking rate is generally programmed at a rate less than
that corresponding to the TARP. This leads to pacemaker Wenckebach
behavior when the patient's atnal rate exceeds the programmed
upper rate (Fig.
16r). The Wenckebach interval is defined as the difference
between the programmed upper rate and the rate corresponding
to the TARP.
Therefore, when a patient has a sinus or other atrial tachycardia,
the pacemaker can track the P waves in a 1:1 fashion up to either
the upper programmed rate of the pacer or to the pacemaker 2:1
rate, which ever is lower. If the 2:1 pacemaker rate is lower,
there may be deleterious hemodynamic consequences for an exercising
patient in whom the ventricular response would abruptly drop
by nearly half. For this reason, a Wenckebach interval is preferred
by programming the TARP to a sufficiently short interval or
the upper rate of the pacemaker to a rate that is less than
the 2:1 AV block rate.
Various strategies are available for active patients with DDD
pacemakers who require physiologic upper rates. Many pacemakers
offer autoadjusting AV intervals that shorten with increasing
rates. By shortening the AV interval, the TARP decreases, which
allows greater upper tracking rates before reaching the rate
of 2:1 AV block. Another strategy involves sensor-driven rate
smoothing. The rate-responsive features are activated, and,
in fact, a separate upper sensor-driven rate, different than
the upper atrial tracking rate, may be programmed. This enables
maintenance of increased ventricular pacing rates driven by
the sensor when the pacer would otherwise respond with AV Wenckebach
or 2:1 AV block.
USE OF PACEMAKERS IN DIFFERENT CLINICAL
SITUATIONS
Paroxysmal Atrial Fibrillation, FLutter,
and Other Tachyarrhythmias
DDD pacing is problematic in the presence
of atrial tachyarrhythmias. During atrial fibrillation, there
are so many sensed atrial events occurring at rapid rates that
a DDD pacemaker responds with an attempt to track these electrograms
up to but not exceeding the upper rate (Fig.
16s and Fig.
16t). The ECG hallmark is an irregularly irregular ventricular
paced rhythm at a mean rate just below the upper rate. Of course,
if the patient has intrinsic AV conduction, the patient's ventricular
rate is not controlled by the pacemaker but rather by the intrinsic
AV nodal conduction.
There are various strategies for preventing inappropriate upper
tracking behavior during atrial tachyarrhythmias. In a patient
with intact AV conduction and paroxysmal atrial tachyarrhythmias,
DDI or DDIR modes would be appropriate (Fig.
16s). In this mode of pacing, there is no tracking of atrial
events. If there is a sinus or other atnal sensed electrogram,
the pacer will inhibit atrial pacing output. Ventricular pacing
occurs only at the lower rate interval. For patients with sick
sinus syndrome, the clinical problem necessitating a pacemaker
is the bradycardia resulting from intrinsic sinus node dysfunction
or the bradyarrhythmias resulting from therapy to suppress the
tachyarrhythmias. Therefore, DDIR is a very effective pacing
mode for patients with sick sinus syndrome who have intrinsic
AV conduction.
At the initiation of atrial fibrillation or other atrial tachyarrhythmia,
many pacers can automatically switch pacer modes from DDD(R)
to VVI(R) or DDI(R) (Fig.
16t ). The automatic mode switch may occur at the upper
rate of the pacemaker or at a separate programmable mode switch
rate. It may occur with single or multiple sequential premature
atrial complexes, depending on the pacemaker model. Mode switching
appears to be a clinically effective method of pacing in patients
with AV block and paroxysmal atrial arrhythmias.
Mode switching reduces symptoms associated with atrial fibrillation
only if patients have adequate control of intrinsic AV conduction
during atnal fibrillation. For this reason, a strategy of AV
junction ablation with implantation of a mode-switching dual-chamber
pacemaker can provide symptomatic relief for those patients
with medically refractory paroxysmal atrial fibrillation with
rapid ventricular response.
Prevention of Atrial Fibrillation by Pacing
The initiation and maintenance of atrial fibrillation
involve several pathophysiologic mechanisms, the most dominant
of which is multiple reentrant pathways (see http://www.rjmatthewsmd.com/
re animation of mechanism of atrial fibrillation). Pacing therapy
may reduce dispersion of refractoriness in the atrium, a feature
in reentry, or eliminate pause-dependent initiation of arrhythmias.
As discussed above atnal-based pacing (AAI or DDD) reduces the
incidence of atrial fibrillation compared with VVI pacing in
those patients who require pacing; it is unknown, however, whether
atrial pacing in itself may reduce the occurrence of atrial
fibrillation. In patients with sick sinus syndrome who require
bradycardia pacing support, it has been suggested that standard
atrial pacing reduces the frequency of atrial fibrillation.
These studies examined the arrhythmia-free interval before and
after atrial pacing. In another study of patients who had atrial
fibrillation without sinus nodal dysfunction, however, DDD pacers
were implanted 3 months prior to planned AV junction ablation,
and these pacers were programmed to DDD pacing at 70 beats per
minute or to backup DDI pacing at 30 beats per minute.The patients
who were actively paced did not have fewer episodes of atrial
fibrillation. Therefore, pacing in itself may not reduce the
occurrence of atrial fibrillation but may be helpful in the
management of those patients who have sinus nodal dysfunction.
It has been reported that dual-site atrial pacing may reduce
the occurrence of episodes of atrial fibrillation. One lead
is placed in the right atrium and a second lead is placed in
the coronary sinus ostium or inside the coronary sinus to advance
left atrial depolarization. In theory, synchronization of the
atria may reduce dispersion of refractoriness and thereby reduce
the occurrence of atrial fibrillation.
Pacing in Chronic Atrial Fibrillation or
Other Atrial Tachyarrhythmia
Patients with persistent atrial tachyarrhythmias
and high-degree or complete AV block generally require a VVIR
pacemaker unless their functional status is limited, in which
case a VVI pacemaker would suffice. DDD(R) may be implanted
in select patients with persistent atrial fibrillation in whom
cardioversion to sinus rhythm is expected.
Pacing in Complete or Intermittent Third-Degree
Atrioventricular Block
Patients with one of the neurally mediated
syncope syndromes generally have intact sinus and AV nodal function.
Because of combined vasodepressor and cardioinhibitory responses,
patients usually require dual-chamber pacing when a pacer is
irnplanted. Additionally, these patients benefit from an interventional
pacing rate (80 to 100 pulses per minute) during their vasovagal
episodes and only require backup pacing at rates of 4O to 50
pulses per minute during other times. Therefore, one algorithm
is to use dual-chamber hysteresis so that when a patient's heart
rate drops to the lower rate, pacing is initiated at the interventional
rate. This algorithm has limitations, since i patient's heart
rate needs to exceed the interventional pacing Lefore inhibiting
the pacer. Some pacemakers now offer rate drop response pacing,
which involves interventional pacing (80 to 110 pulses per minute
with gradual decline in paced rate at 1 to 5 minutes) that is
triggered by a steep drop in a patient's intrinsic heart rate.
Based on the North American Vasovagal Pacing Study, there was
a reduction in syncope from 70 percent in the control group
to 22 percent in patients who had pacers implanted with the
rate-drop response feature.
Pacing in Cardiac Transplant Patients
After orthotopic cardiac transplant, there
is a high incidence of chronotropic incompetence resulting in
slow junctional rhythm, sinus arrest, or sinus bradycardia.
Bradycardia tends to resolve spontaneously in most patients,
but 6 to 21 percent of patients may require permanent pacing.
Although symptomatic bradycardia is generally an early finding
after transplantation, up to 5 percent of patients following
transplant may have symptomatic bradycardia as a late finding.During
the implant, the atrial lead is positioned in the donor atrium.
A DDDR or AAIR pacer is placed, depending on whether AV conduction
is intact.
HEMODYNAMICS OF CARDIAC PACING
In theory. a pacemaker optimizes and maintains
AV synchrony and optimizes ventricular activation and heart
rate to enable cardiac output to meet the metabolic needs of
the patient, whether he or she is resting, sleeping, or exercising.
There are many variables involved in determining cardiac output
through an effect on stroke volume, such as the autonomic tone,
physical condition of the patient, left ventricular diastolic
and systolic function, and peripheral vascular resistance. As
seen in Table 6, many variables in pacing systems can affect
cardiac hemodynamic function.
TABLE
6 Effects of Cardiac Pacing Variables on Hemodynamic Function
Atrioventricular Interval
The role of the AV interval and the optimal
AV interval for improving hemodynamic function has been studied.
For most patients, the optimal AV interval corresponds to the
physiologic range (i.e., an AV interval of approximately 150
± 50 ms). In clinical practice, however, most patients'
quality of life is not significantly different between AV intervals
that are optimized by noninvasive assessment versus AV intervals
that are suboptimal.
There are other considerations when programming AV intervals.
With AV sequential pacing, the start of the P wave corresponds
to the start of the AV interval while, with P-wave synchronous
ventricular pacing, the start of the P wave begins approximately
20 to 70 ms prior to the start of the AV interval, depending
on the conduction time from the sinus node to the atrial electrodes.
The optimal AV interval for P-wave synchronous ventricular pacing
would be shorter than the optimal AV interval for AV sequential
pacing."2 Therefore, to achieve similar hemodynamic effects
from ventricular pacing following a sensed or paced P wave,
the sensed AV interval should be programmed approximately 40
to 50 ms shorter than the paced AV interval. Additionally, left
ventricular cardiac function is more dependent on left atnal
to left ventricular relationships rather than right atrial to
right ventricular AV interval. For this reason, there is much
variability between patients with respect to programming AV
intervals.
Pacemaker Syndrome
The pacemaker syndrome is a constellation
of signs and symptoms representing adverse reaction to VVI pacing.
Most of the symptoms relate to loss of AV synchrony and also
to retrograde conduction. These include orthostatic hypotension,
near syncope, fatigue, exercise intolerance, malaise, weakness,
cough, awareness of heartbeat, chest fullness, neck fullness,
headache, chest pain, and other symptoms that may be nonspecific.
On exam, these patients may have intermittent or persistent
cannon A waves and possible liver pulsation. ECG demonstrates
VVI pacing present at the time of the symptoms.
The basis for pacemaker syndrome is not only loss of AV synchrony
but also the presence of ventricular-atrial conduction. Atrial
contraction against closed AV valves leads to increases in jugular
and pulmonary venous pressure causing cough and m alaise in
patients with intact cardiac function and congestive heart failure
in other patients with structural heart disease. Distended atria
can lead to reflex vasodepressor effects mediated by the autonomic
nervous system and diuresis mediated by elevated levels of atnal
natriuretic malaise in patients with intact cardiac function
and peptide. Therefore, if patients have decreased cardiac output
and arterial pressure secondary to VVI pacing, autonomic and
humoral reflexes can lead to further hypotension and hemodynamic
deterioration.
DDI pacing may produce pacemaker syndrome if the sinus rate
exceeds the lower rate. DDD pacing can lead to pacemaker syndrome
in select patients with severe intraatrial conduction delay
who experience inappropriate timing between left atrial systole
and left ventricular contraction. This may necessitate the addition
of a coronary sinus pacing lead to advance left atrial systole.
The management of pacemaker syndrome usually requires restoration
of AV synchrony. In many patients, an upgrade to a dual-chamber
pacer is indicated. In some patients with intact sinus and AV
conduction, lowering the pacing rate in VVI mode and using the
hysteresis mode may promote sinus rhythm, lessening the symptoms
associated with pacemaker syndrome. Using the VVIR mode by itself
will not prevent or reduce symptoms from the pacemaker syndrome.
Many patients may experience mild symptoms of the pacemaker
syndrome and not recognize the symptoms until after an upgrade
to a dual-chamber pacemaker. Most patients prefer DDD pacing
to VVI pacing in various clinical and hemodynamic studies.
RATE-RESPONSIVE PACEMAKERS
The ability of a pacemaker to increase the
lower rate in response to a physical or physiologic stimulus
is termed rate-responsive, rate-adaptive, or sensor-driven pacing.
The letter R in the fourth position of the NASPE/BPEG pacing
code indicates rate-responsive pacing. Sensor systems that respond
to parameters or activities that correlate with physiologic
need for increased cardiac pacing rate provide input to the
pacer, which increases the pacer lower rate. Numerous sensors
have been developed with the goal of providing sensor input
into the pacemaker, which can be then used to provide rate-adaptive
pacing.
Hemodynamic Evaluation of Rate-Adaptive
Pacing
Cardiac output is a function of ventricular
rate and stroke volume, modified by variables such as AV synchrony,
ventricular preload, ventricular afterload, and autonomic state.
In normal individuals at rest, pacing-induced increase in ventricular
rate usually results in a transient increase in cardiac output
followed by decrease in stroke volume, returning cardiac output
toward normal. When there is a physiologic need for increased
cardiac output, however, such as during exercise, stroke volume
is maintained during increased ventricular pacing rate.
The role of the atrium and the need for AV synchrony remain
less certain during faster rates compared with heart rates under
100 beats per minute. In patients with AV and ventricularatrial
block, pacing in the VDD mode compared with VVI pacing matched
to the atrial rate (without AV synchrony) appears to provide
similar cardiac output. Multiple studies have shown that the
change in work capacity correlates with ventricular rate during
exercise whether the ventricular rate is triggered by spontaneous
atrial activity or by a pacemaker sensor. Therefore, AV synchrony
may be less important in patients during exercise who achieve
or require heart rates in excess of 120 beats per minute. Nevertheless,
VVIR pacing is not a substitute for DDD pacing.
If a patient has a VVI pacemaker and ventriculoatrial conduction,
or a DDD pacing programmed with long AV intervals such that
the P wave is closer to the preceding R wave, deleterious hemodynamic
consequences may result. In this circumstance, there would be
a decrease in cardiac output, since the atrium would consistently
pace against closed AV valves, producing increases in the pulmonary
and jugular venous pressures. This would also produce symptoms
of the pacemaker syndrome. Dual-chamber pacemakers currently
available often have options of rate-adaptive AV intervals.
This provides the advantage of maintaining normal AV relationships
during exercise and prevent retrograde atrial contraction.
RATE-ADAPTIVE SENSORS
Multiple rate adaptive sensors are available
or under development. Actively based sensors are used most commonly.
These are piezoelectric crystal systems that are very sensitive
to detection of vibration induced by up-down motion (activity)
or acceleration, particularly (forward-backward motion).
The drawback of activity-based pacers is that they do not provide
feedback that is proportional to physiologic need. For instance,
climbing up stairs requires more work than going down stairs;
however, going down stairs is usually faster and would activate
the sensor more than climbing up stairs. This leads to faster-paced
rates while going down stairs. Similarly, other activity with
little body vibrations may produce ineffective rate adaptation
from the pacemaker. Therefore, true physiologic sensors are
desirable for rate-responsive pacing. The role of physiologic
sensors is to provide some measurable index of activity, exercise,
or catecholamine state that can provide a more accurate input
to the pacemaker for rate-adaptive pacing. The QT interval is
affected by heart rate but also independently by catecholamines.
Therefore, pacers can measure the interval from the ventricular
stimulus to the end of the sensed T wave and modulate heart
rate based on this measurement. The drawback of this technique
is that the patient has to be ventricular paced in order to
measure the QT, or stimulus-T, interval.
Since there exists a close relationship between respiratory
rate or minute ventilation and heart rate, various sensors incorporate
measurements of respiratory effort. These systems are based
on measurement of transthoracic impedance between the pacemaker
lead and the pulse generator. The impedance increases with inspirations
and decreases with expiration; the amplitude of the impedance
change is proportional to the tidal volume. Minute ventilation
is the product of the tidal volume and respiratory rate. Thus,
minute ventilation can provide an accurate physiologic estimate
of metabolic needs. One of the disadvantages of this system
is that energy is required to measure impedance, which increases
current drain from the pacemaker.
A number of other sensor systems are available or under development.
Many use physiologic parameters, such as pH, oxygen saturation,
stroke volume, or temperature. The premise behind all of these
are that the measured parameters can provide an accurate measure
of a patient's metabolic needs, which can be used to guide rate
responsiveness. There are various benefits and drawbacks to
the different methods.
DUAL SENSORS
Some sensors systems provide the advantage of more physiologic
pacing during steady state but have a slow response time during
initiation of exercise. Other sensors, particularly activity
sensors, have fast response times at initiation of activity
but may not produce physiologic responses during peak or steady-state
activity. Pacers with dual sensors can provide patients with
rapid responses during the start of exercise to augment the
heart rate and a more physiologic sensor (QT, minute ventilation)
to provide more proportional heart rate response during steady
state. The benefit of dual sensors has not been conclusively
demonstrated in long-term randomized studies, and, in fact,
one acute exercise study demonstrated no clinical advantage
of dual sensor over single-sensor rate-responsive pacing.
Programming Rate-Adaptive Parameters
The parameters for programming rate responsiveness
include the lower and upper activity rates, which may be separate
from the upper tracking rate. A treadmill test may be required
to optimize pacemaker programming. In practice, it is often
sufficient to have the patient walk for a few minutes and program
the rate-responsive features to achieve what would be expected
to be a physiologic pacing rate for that patient. Different
pacers have different algorithms that can automate the adjustments
of the rate responsiveness. In most patients, it is difficult
to demonstrate clinical effectiveness of automatic rate-response
optimization versus fixed rate-responsive programming in the
office or clinic.
PACEMAKER COMPLICATIONS
Pacemaker complications can occur at the time
of implantation or, less likely, can occur late after implantation
(Table 7). Overall, early complications have been reported in
the range of 3 to ii percent, depending on the definition of
complication, duration, and intensity of follow-up.
TABLE 7 Complications Related
to Pacemakers

Complications of Pacemaker Implant
Cardiac perforation is a potentially serious
and often unrecognized complication of pacemaker lead insertion.
This may be recognized at the time of lead insertion by fluoroscopic
position of the lead, a paced QRS complex having right bundle
branch block pattern, diaphragmatic stimulation, or hypotension
resulting from cardiac tamponade. In the absence of anticoagulation,
perforation usually does not lead to tamponade if the lead is
withdrawn and repositioned. After implantation, cardiac perforation
may be recognized by pericardial pain, friction rub, increasing
ventricular pacing threshold, diaphragmatic stimulation, or
pericardial effusion. The presence of these signs is not diagnostic
of cardiac perforation, and echocardiograms should be performed
to examine the lead position. If perforation is suspected, and
the patient is hemodynarnically stable, clinical observation
is often the prudent course.
Other implant-related complications include subclavian arterial
puncture, pneumothorax, hemothorax, and air embolus. Rarely,
a lead may be introduced into the left ventricle through an
inadvertent subclavian arterial puncture or through an unrecognized
atrial or ventricular septal defect.
Complications of venous leads include venous occlusion with
resulting superior vena cava syndrome or thrombosis of the subclavian
vein with ipsilateral arm edema. Acute thrombosis may be treated
with heparin and warfarin and managed conservatively if the
patient responds to anticoagulation. Invasive and surgical interventions,
including venoplasty and stent placement, have been described.
Most occlusions, partial or complete, may occur over time and
tend to be asymptomatic because of the formation of venous collaterals.
Infections related to pacemaker implantation are rare. The use
of prophylactic antibiotics and irrigation of the pacemaker
pocket with antibiotic solution may help prevent infection,
especially from local flora. Early infections may be caused
by Staphylococcus aureus and can be aggressive. Late infections
are commonly related to Staphylococcus epidermidis and may have
a more indolent course. Occasionally, pacemaker infections are
misdiagnosed as pacemaker allergy. Other signs of infection
include local inflammation and abscess formation, erosion of
the pacer, and fever with positive blood culture without an
identifiable focus of infection. Transesophageal echocardiography
may help determine whether vegetations are present on the pacemaker
lead. If the pacemaker is infected, removal of the pacemaker
leads and generator is usually required.
Mechanical Complications
During implant, the leads are connected to
the pulse generator by a setscrew mechanism. If the setscrew
is loose (Fig.
16d), then pacemaker malfunction may occur, manifested by
increased impedance and intermittent or complete failure to
capture.
The pacemaker leads are subject to long-term complications.
The insulation of the leads may break, leading to problems with
oversensing (due to electrical noise), undersensing, and failure
to capture (due to current leak). This problem often manifests
intermittently and may be difficult to detect during a routine
pacer check. The patient may complain of pectoral muscle stimulation
due to current leak around an insulation break. An abnormally
low impedance with demonstrable lead malfunction is diagnostic
for insulation break. Subtle insulation breaks may be detected
by having the patient perform provocative maneuvers while monitoring
an ECG (and marker channels) and or measuring impedances.
Leads may also fracture over time (Fig.
16b). Early lead fractures lead to increased impedances
associated with failure to capture, oversensing, and undersensing.
Some leads use retention wires to preform an atrial lead so
that it is more likely to attach and remain within the atrial
appendage. Fracture of a retention wire does not cause any pacemaker
malfunction, but it can lead to serious complications, including
cardiac perforation and death, when it penetrates through the
insulation into the atrial cavity.
Twiddler's syndrome is a term applied
to patients who intentionally or unintentionally manipulate
their pulse generator, causing twisting of the entire pacemaker
system. This leads to lead dislodgment or fracture. This may
also result from an excessively large pacemaker pocket allowing
rotation of the pacemaker.
Electromagnetic Interference of Pacemaker Function
In general, electromagnetic interference (EMI)
can originate from a variety of sources that have the potential
to affect pacemaker function adversely. In Table 8 are listed
some of the more common sources of EMI with potential pacemaker
effects.
TABLE 8 Sources of Electromagnetic Interference
and Potential Effects
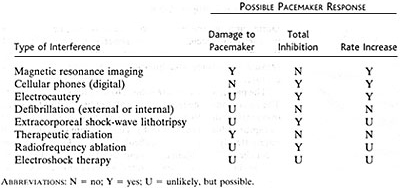
Unipolar pacemakers are usually more susceptible
to EMI interference than are bipolar pacemakers because the
sensing circuit encompasses a larger area compared with bipolar
sensing. Factors that affect EMI interference have to do with
the source of the interference and the proximity to the pacemaker
generator. Many of these sources are located in a hospital environment
or specialized places such as construction sites. Magnetic resonance
imaging scans are contraindicated in patients with pacemakers,
although there are case reports of patients with pacers undergoing
MRI scans without adverse events. Sources of EMI at home and
the office usually do not pose a problem for patients. There
is concern, however, that electronic article surveillance devices,
found commonly in retail establishments, can interfere with
pacemaker function, if patients linger by these devices.
The effects of EMI vary according t o its source and the type
of pacemaker. Inhibition of pacing output can potentially be
life threatening for patients who are pacemaker dependent. If
the EMI is interpreted as atrial events by the pacemaker, then
inappropriate ventricular pacing may occur in patients with
DDD pacemakers, since these pacemakers attempt to track these
events, which are interpreted as atnal. EMI often causes electrical
noise that causes the pacemaker to function in a noise reversion
mode. The actual function of this mode differs among the different
pacemakers, but this mode involves switching to an asynchronous
pacing mode. After elimination of this interference, pacers
generally revert to the previously programmed mode; however,
it is possible for EMI to cause pacemakers to revert to a backup
pacing mode. Backup pacing in some models is unipolar VVI pacing
at a preset rate.
Occasionally, EMI causes permanent damage to the pulse generator.
Therapeutic radiation can damage the complementary metal oxide
semiconductors (CMOS) that are part of most modern pacemakers.
Generally, doses in excess of 5000 rad, but as little as 1000
rad, may induce pacemaker circuitry damage, which in turn can
cause pacemaker failure or even induce a runaway pacemaker.
If the pacemaker cannot be shielded from the field of radiation,
then consideration should be given to reimplanting the pacemaker
at a distant site.
In studies examining interactions between pacemakers and cellular
telephones, it was noted that digital telephones may cause intermittent
pacemaker dysfunction. These adverse effects observed included
pacemaker inhibition, inappropriate ventricular tracking (in
VDD or DDD pacemakers), or resetting the pacemaker to a backup
asynchronous mode. Factors associated with interference include
unipolar pacing systems, digital cellular phones, increased
output by the cellular phone, and close proximity of the cellular
phone to the pacer. Because of the diversity of cellular phones
and pacemakers that have different shielding capabilities against
electromagnetic interference, it is difficult to draw firm conclusions
on the use of digital cellular telephones.159 No consistent
problems have been detected with analog telephones. It is advisable
that patients use cellular telephones that are analog or to
keep digital cellular (with power outputs greater than 3 W)
phones 20cm away from their pacemaker generator.
Medtronic Standard GuideLines Regarding Electrosurgery
and Pacemakers
Message of 9/11/01 Regarding Phacoemulsion
Procedures and Pacemakers:
Regarding phacoemulsification procedures and pacemaker dependent
patients this procedure should pose no problems given that the
energy is sound waves and not generation of an electric current.
ELECTROSURGERY AND PACEMAKERS
Essentially, any type of interference has
the possibility of causing reversion to asynchronous pacing
or possibly pacemaker inhibition. Interference could cause complete
inhibition. Electrosurgery and defibrillation have the potential
to cause either of these things to happen.
Since electrosurgery and especially defibrillation
are generally necessary procedures, Medtronic makes recommendations
on how to minimize the possible pacemaker interference problems.
The remainder of this letter will contain these recommendations.
Effects of electrosurgical currents upon implanted
pulse generators can be controlled by the location of the electrosurgical
electrode in respect to the implanted pacemaker system, the
orientation of the surgical electrodes to the pacing system,
the spacing of the surgical electrodes from the pacemaker, and
the frequency of application of the electrosurgical currents.
I will describe these four areas first and then discuss other
factors which I feel may influence how electrosurgery is used.
If possible, the return or ground electrodes
should be placed so that the pacing system is not directly in
the current path between the active and return electrodes on
the arm, leg, neck, etc., may help to position the electrodes
away from the pacing system.
Electrosurgical effects are reduced if the
current is flowing perpendicular to the electrodes of the pacemaker
lead. In a bipolar pacemaker, the orientation of the pacing
lead electrodes within the heart may be difficult to determine.
A unipolar pacing system orientation is easier to determine
with one pacing electrode in the heart while the pulse generator
itself acts as the indifferent electrode. Unipolar systems are
more susceptible to external interference due to the larger
"antenna effect" they present. It is more important
to have a perpendicular current flow past the electrodes of
a unipolar pacing system than in a bipolar system.
Very high current densities are generated
at the active electrosurgical electrode. Placement of this electrode
near the implanted pulse generator may induce sufficient current
to the pulse generator to cause it to revert to a fixed rate
or inhibit. Placement of the active electrode as far away as
possible from the pulse generator will minimize the effects
of these induced currents. Currents at the active electrode
disperse rapidly so that at a distance of only a few centimeters
the effects on the pulse generator will be substantially reduced.
Pulsing modes of interference typically have
more effect on pulse generators than do continuous sources.
Pulses may be generated by turning the electrosurgical equipment
on and off or by removing and reapplying the active electrode
while the equipment is turned on. This type of effect can be
reduced by minimizing the number of times the surgical equipment
is activated. If inhibition is suspected, use of electrosurgery
should be limited to one second with a rest period of approximately
10 seconds. This procedure allows the pulse generator to function
properly for a greater portion of the time, therefore, permitting
the patient's cardiac output to be properly maintained.
Excessive power usage also contributes to effects upon pulse
generators. Maintaining equipment in good working conditions
will help reduce the power required and also minimize the emission
of spurious interference.
Placing a magnet over a demand nonprogramrnable
pulse generator will cause it to operate asynchronously at a
fixed rate. This will also eliminate the effects of external
interference by disabling the sensing amplifier of the pulse
generator. If the patient can tolerate the competitive pacing
of asynchronous pacing, then I would suggest placing the magnet
over a nonprogrammable demand pulse generator when using electrosurgical
currents.
Do not place a magnet over a demand programmable
pulse generator when an external source of interference is present.
The magnet will cause the pulse generator to operate asynchronously,
but also activates the programming circuitry. Reprogramming
may occur if the external interference is of sufficient amplitude
to affect the pulse generator while the programming circuit
is activated by a magnet. Reprogramming will not occur as readily
in the new multiprogrammable pulse generators. These units require
a special digital series of codes before access can be made
to the programming circuitry.
Inadvertent touching of the active electrode
to the implanted pulse generator may result in excessive current
conducted into the pacing lead system. Repositioning of the
lead may be required if tissue is damaged at the pacing electrode
site.
In summary, unipolar pacing systems are more
susceptible to external interference. Being aware of the location,
orientation, spacing, and application procedure of the electrosurgical
electrode may help minimize effects on the pulse generator.
Do not use a magnet over a programmable demand pulse generator
in the presence of strong interference fields. Proper maintenance
and use of power of the surgical equipment will reduce undesirable
effects on the pulse generator.
Defibrillation concerns for implantable pulse
generators are very similar to those stated for use with electrosurgery.
If possible, position the electrodes so that currents are not
passing through the pacing system. Place the defibrillator electrodes
at least thirteen centimeters or five inches from the implanted
pulse generator and use the least amount of energy to satisfactorily
revert the patient. Repeated discharges to the implanted pulse
generator will not have the cumulative damaging affects.
lmplantable Medtronic pulse generators
are designed to withstand 400 watt-seconds of defibrillation
energy. The functional operation of the pulse generator may
be verified following defibrillatory discharges.
Rev. 10.95
ELECTROSURGERY RECOMMENDATIONS
1. Do not perform electrosurgery within six
inches of the pulse generator.
2. Use the minimum electrosurgical power settings
required.
3. Use short bursts (preferably less than
one second in duration) spaced more than five seconds apart.
If electrosurgery is causing inhibition of the pacemaker, a
longer time between bursts will minimize hemodynamic effects.
4. If the patient can tolerate asynchronous
pacing, the pacemaker mode can be changed to either VOO or AOO.
In the case of a nonprogrammable pacemaker, simply place a magnet
over the IPG. Do not place a magnet over a programmable pulse
generator, rather program the pacemaker to asynchronous operation
prior to surgery. Asynchronous operation eliminates the potential
for reversion or inhibition due to oversensing.
5. If possible, a bipolar cautery should be
used. A bipolar system has a definite, short current path which
greatly reduces the area of interference to roughly a six-inch
circle centered around the electrosurgical site. If a unipolar
device is used, the indifferent electrode should be placed such
that the current flowing between the electrosurgical site and
the indifferent electrode (ground plate) will not intersect
the pacing system.
6. Always monitor pacemaker patients during
electrosurgery. If, because of interference, the ECG tracing
is not clear, the patients pulse generator should be monitored
manually or by some other means such as ear or finger plethosgraphy,
Doppler pulse detection, or arterial pressure display.
7. Emergency pacing equipment as well as an
appropriate programmer should be readily available.
8. Verify function of IPG after procedure.
Remember to reprogram out of VOOIAOO modes if that was done
prior to the procedure.
Rev. 10.95
PACEMAKER MALFUNCTION
Pacemaker malfunction can be categorized as
loss of capture, abnormal pacing rate, undersensing, oversensing,
or other erratic behavior. The approach to diagnosing pacemaker
malfunction is to inspect the ECG carefully, interrogate the
pacemaker; check pacing and sensing thresholds, lead impedances,
and battery voltage/ magnet rate; and perform a chest x-ray.
Many instances of pacemaker malfunction actually represent normal
function of the pacemaker (Table 9). Usually, causes of pacer
malfunction may be diagnosed noninvaasively, but, occasionally,
surgery is required to diagnose problems..
TABLE
9 Suspected Malfunction Occurring During Normal Pacer Function
TABLE 10. Causes of Upper Rate Behavior
in DDD Pacemakers (and Atrioventricular Block)
| |
ECG Characteristics |
Response to Magnet |
| Sinus tachycardia |
1:1 Atrioventricular pacing,
pacemaker Wenckebach or 2:1 block depending on the PVARP,
upper rate, and sinus rate |
No change in paced rhythm
after magnet removed |
|
Atrial fibrillation |
Irregularly irregular paced ventricular rhythm up to but
not exceeding the upper rate |
No change in paced rhythm
after magnet removed |
|
Pacemaker-mediated |
Regular paced ventricular rhythm equal to or less than
upper rate |
Termination of tachycardia |
ABBREVIATION: PVARP =
postventricular atrial refractory period.
Abnormal Pacing Rates
Abnormal pacing rates can be due to normal
or abnormal pacing function (Table 9). Failure of the pacemaker
to output is usually due to oversensing. Occasionally, there
is pacemaker output that is not visible because of bipolar pacing
producing very low amplitude pacing artifacts (artifacts from
digital ECG recording are commonly difficult to visualize).
Conversely, absence of pacing stimuli may be due to interruption
of current flow from a lead fracture, insulation break, or a
loose setscrew.
Abnormally fast pacing rates usually are due to normal pacing
function. They may be in response to rate-adaptive sensors.
In DDD pacemakers, upper rate pacing may be due to sinus tachycardia,
atrial tachyarrhythmias, or pacemaker-mediated tachycardia (Table
10). In ei ther case, the pacemaker function is normal and is
responding either to a rapid atrial rate or to retrograde atrial
activity. Rarely, very rapid ventricular pacing ("runaway
pacemakers") can cause life-threatening problems requiring
disconnection of the pacemaker. Occasionally, abnormal pacing
rates can be due to an unstable lead position where the lead
is swinging between heart chambers.
Loss of Capture
The loss of pacemaker capture occurs
when there is a visible pacing stimulus and no atrial or ventricular
depolarization. This may be intermittent or persistent. Most
problems occur at the pacemaker lead/tissue interface. For instance,
lead dislodgment can cause obvious failure to capture. An increase
in the pacing threshold above the pacing output can occur as
part of the rise above initial threshold within a few weeks
following lead placement (Fig.
16u) or because of drug therapy, electrolytes, myocardial
infarction, or ischemia. Fracture of the lead, insulation breaks,
and loose setscrews are mechanical problems that can cause failure
to capture. Lastly, battery depletion may cause the pacing output
to decline sufficiently such that pacing failure occurs.
Loss of capture requires a check
of pacing threshold and of pacing lead impedance and a chest
x-ray. For instance, if the problem is an elevated pacing threshold,
pacing outputs must be increased. Abnormal lead impedances may
confirm a lead failure and the need for lead replacement.
Oversensing
This problem leads to abnormal pacing
rates with pacemaker pauses. Generally, unipolar lead systems
are more susceptible to oversensing. The sources for oversensing
can be intracardiac, extracardiac, or due to EM!. Analysis of
ECG, especially with pacemaker interrogation and pacemaker marker
channels, may help to determine the cause. If the oversensing
is regular, analysis of the pauses may suggest T-wave or P-wave
oversensing. T-wave oversensing usually can be eliminated by
decreasing the sensitivity (increasing the millivoltage required
to sense electrical activity) or increasing the ventricular
refractory period.
Oversensing due to lead fracture, insulation break, or other
electrode problems will usually be random and erratic (Fig.
16v). With early lead problems, the malfunction is intermittent
and may be exacerbated by certain body positions or motions.
In later stages, the combination of oversensing, undersensing,
and failure to capture is almost always diagnostic of a lead-related
problem. Programming to an asynchronous mode may temporarily
control this problem while awaiting a lead replacement, which
should be carried out as promptly as possible.
Crosstalk inhibition is a phenomena usually seen in unipolar
pacers. It is due to ventricular sensing of atrial output. This
is currently a rare problem because UI blanking periods 41IU
ventricular safety pacing.
Myopotential oversensing is usually a problem in umpolar but
not bipolar systems. These skeletal myopotentials generate interference,
which tends to correspond to certain activity. The optimal solution
is reprogramming the sensitivity to a level high enough to avoid
myopotential sensing while preserving adequate safety margin
to sense intrinsic cardiac depolarizations.
Undersensing
An inadequate intracardiac signal can
lead to undersensing (Fig.
16q). The intracardiac electrograms can deteriorate due
to inflammation or scar formation at the tissue lead interface.
Additionally, certain drugs, electrolyte abnormalities, infarction,
ischemia, lead fracture, or insulation breaks can lead to undersensing.
Cardioversion or defibrillation can also cause attenuation of
intracardiac electrograms.Usually,undersensing is a greater
problem in the atrium than in the ventricle. The optimal solution
is to program an enhanced sensitivity (decrease sensing level).
With bipolar systems, the programmed sensitivity can usually
be reduced to 0.18 mV in the atrium, without oversensing of
myopotentials or other extraneous signals.
Other etiologies for undersensing occur when intrinsic atrial
or ventricular complexes fall within one of the programmed refractory
periods. Undersensing can also result from a pacer that was
inadvertently programmed to an asynchronous mode (occasionally
occurring with battery depletion or pacemaker generator reset).
Mitrani,R.D. and others,Cardiac Pacemakers,Hurst's
The Heart,10th edition,Vol.1,pp.963-992 .