Due
to major advances in both diagnosis and treatment of congenital
heart disease in children, many are living into adulthood. There
are almost one million such patients this year.
Congenital
heart disease can be divided into two types:
1.
The acyanotic ones in which the oxygen level in the blood is
high enough to keep the patients' color pink;
2.
The cyanotic ones in which the oxygen level in the blood is
low enough for the lips and skin to show varying degrees of
bluish discoloration.
1.
Acyanotic congenital heart consist of the following:
a) atrial septal defect ( figures 112a, 112b )
b) ventricular septal
defect ( figure 112c )
c) patent
ductus arteriosus ( figure 22 )
d) aortic stenosis ( figures 24a, 24b, 46a, 46b, 46c, 47 )
e) pulmonary stenosis ( figure 25a, 25b )
f) parachute
mitral valve ( figures 44g-1 and 44g-2 )
g)
coronary artery fistula
h) anomalies of the great veins
Brickner,M.E.
and others,Congenital Heart Disease inAdults,N.Engl.J.Med.,Vol.342.N.4,Jan.27,2000
2.
Cyanotic Congenital Heart
Disease features bluish discoloration
of the skin and lips as opposed to the normal pink appearance.
The cyanosis is due to the shunting of systemic venous blood
to the arterial circulation causing arterial blood desaturation
of oxygen. The size of the shunt determines the degree of desaturation.
In adults the most common causes of cyanotic congenital heart
disease are tetralogy of Fallot and Eisenmenger's syndrome.
a) Tetralogy
of Fallot ( figure 23d
)
b) Ebstein's Anomaly
( figure 23e
)
c) Transposition
of the Great Arteries ( figure 23h
)
d ) Eisenmenger's syndrome
( figure 23j
) Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
e) Hypoplastic
Left Heart Syndrome
a)
Tetralogy of Fallot
It
is characterized by a large ventricular septal defect (VSD,
figure 112c),
an aorta that overrides the left and right ventricles, obstruction
of the right ventricular (RV) outflow tract, and RV hypertrophy
(increased wall thickness). As obstruction in RV outflow tract
increases, more blood is shunted through the VSD to the left
side of the heart to cause more cyanosis (see figure 23d).
Increases in resistance to flow in the general arteries of the
body causes less shunting, and decreases cause more shunting
to the left.
Symptoms
in adults include shortness of breath and limited exercise tolerance.
Complications include brain abscesses, strokes and heart infections
(see figures 48a,
48c,
48d).
Such patients may have enlargement of the distal ends of their
fingers called clubbing. Most patients without surgical correction
die in childhood.
Echocardiography
can establish the diagnosis. Color Doppler can visualize the
VSD. Heart catherterization can confirm the diagnosis.
Surgical
repair is recommended to relieve symptoms and to improve survival.
Complete surgical correction (closure of the VSD and relief
of RV outflow obstruction is performed currently when patients
are very young. Patients are at risk for heart infections and
should thus receive prevention with antibiotics before dental
or elective surgical procedures.
Even
with repair these patients have a poorer survival rate (apparently
due to cardiac causes such as arrhythmias) than that of an age-matched
control population. Ventricular arrhythmias can be detected
with Holter monitoring in 40 to 50 percent of patients with
repaired tetralogy and are most likely to occur in patients
who are older at the time of surgical repair and those with
moderate or severe pulmonary regurgitation,systolic and diastolic
ventricular dysfunction, prolonged cardiopulmonary bypass, or
prolongation ot the QRS intreval (to greatwer than 180msec).
Patients with repaired tetralogy of Fallot often have atrial
fibrillation or flutter, which may cause considerable morbidity.
Patient
with repaired tetralogy are at risk for other chronic complications.
Pulmonary regutgitation may develop as a consequence of surgical
repair of the right ventricular outflow tract. Although even
substantial regurgitationcan be tolerated for long periodds,
enlargement of the right ventricle eventually occurs, with resultant
right ventricular dysfunction, and repair orreplacement of the
pulmonary valve may be in required. An aneurysm may form at
the site where the right ventricular outflow was repaired;rupture
has occurred rarely.
Alternatively,
patients may have residual or recurrent obstruction of the right
ventricular outflow tract, requiring repeated surgery. Approximately
10 to 20 percent of patients with repaired tetralogy of Fallot
have residual ventricular septal defects, and such patients
may require repeated sirgery if the defects are of sufficient
size. RBBB is common after repair of tetralogy of Fallot, but
complete heart block is rare. Finally, aortic regurgitation
may occur but is usually mild.
Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
Late survival is excellent, even in
patients who underwent repair during the very early years of
open heart surgery. Surgery can not be considered curative,since
survival,even in excelllent series is slightly but significantly
worse than for a matched control population. The risk factors
for an adverse late outcome include older age at surgery, preopoerative
congestive heart failure, a previous Potts operation, persistent
right ventricular systolic hypertension, and a residual ventricular
defect. Late death may be sudden, due to tachyarrhythmias or,
very rarely in the current era to conduction disease. Left and
right ventricular failure due to right ventricular overload
or left ventricular volume overload is another important cause
of late death in older patients.
The late functional outcome is excellent for the mayority of
patients. Most live normal lives, but the results appear to
be better in those undergoing surgery at a younger age. Pulmonary
valve replacement can be accomplished with low risk.
Exercise performance is usually impaired when surgery is undertaken
in adolescence or adulthood.
b)
Ebstein's Anomaly
This
anomaly is due to a defect in the tricuspid valve (TV) with
the septal and posterior leaflets displaced down into the right
ventricle, while the anterior leaflet is malformed and abnormally
attached to the RV free wall (see figure
23e). This valve often allows blood to regurgitate from
the small RV back into the large RA.
Eighty percent of these patients have ASD's through which right-to-left
shunting of blood may occur with cyanosis. Such patients are
at risk for a paradoxical embolus (blood clot) from the RA through
the LA to the brain with abscess(instead of the normal route
of an embolus from the legs to the lungs via the right ventricle
through the pulmonary valve)and sudden death.
There
is usually a heart murmur. EKG abnormalities are often present
including WPW syndrome, an atrial tachycardia or rapid heart
beat (see figures
2, 3a).
Twenty percent have an accessory electrical pathway between
the atrium and ventricle (see figure
1) to account for the cardiac arrhythmias.
An echocardiogram
can define the abnormalities, and a color Doppler imaging study
can determine the presence and size of interatrial shunting.
Management
involves prevention of complications, such as heart infection,
prevented with antibiotic prophylaxis. Heart failure is treated
with diuretics (diuril, lasix, etc) (to eliminate fluid) and
digoxin (a heart drug to improve heart muscle contractions).
Arrhythmias may be treated with medication or catheter ablation
(see figures 3b,
11).
Repair
or replacement of TV in conjunction with closure of the interatrial
communication is recommended in older patients with severe symptoms
despite medical therapy and heart enlargement.
Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
c)
Transposition of the Great Arteries
In
d-transposition of the great arteries, the aorta arises in an
anterior position from RV and the pulmonary artery arises from
LV (see figure 23f).
In two thirds of cases the ductus arteriosus (see figure 22)
and foramen ovale allow communication between the aortic and
pulmonary circulations. Severe cyanosis is present. The one
third with other defects that permit intracardiac mixing (i.e.
ASD figures 112a
and 112b,
VSD figure 112c, PDA figure 22)
are less critically ill with loss of severe cyanosis, but they
are at risk of LV failure.
Findings
include cyanosis and heart murmur. RVH (increased RV wall thickness)
or LVH (increased LV wall thickness) may be present. Chest X
ray shows heart enlargement.
Immediate
management involves creating intracardiac mixing or increasing
its extent:
1) use of infusing of medication, prostaglandine E, to maintain
or restore patency of ductus arterioses, 2) the creation of
an ASD or both.
Also, oxygen is administered to most patients (to decrease pulmonary
[lung] vascular (blood vessel) resistance and to increase lung
blood flow), as are digoxin and diuretic drugs like diuril or
lasix (to treat heart failure).
Two
surgical operations have been used (see figure
23f regarding the atrial switch operation). The atrial switch
operation as shown in figure 23f
has been replaced by the arterial switch operation in which
the pulmonary artery and ascending aorta are transected above
the semilunar valves and coronary arteries (see figure 23i),
and then switched, so that the aorta is connected to the neoaortic
valve (formerly the pulmonary valve) arising from the left ventricle
(LV), and the pulmonary artery is connected to the neopulmonary
valve (formerly the aorta valve) arising from the RV (see figure
23i).
The coronary arteries are relocated to the neoaorta to restore
normal coronary circulation. This operation can be performed
in neonates (newly born) and is associated with a low operative
mortality and an excellent long-term outcome.
Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
The Arterial Switch Operation
Surgical Repair of d-Transposition
of the Great Vessels Arterial switch operation for d-TGA with
intact ventricular septum
Cardiopulmonary bypass can be conducted
in a number of ways, depending on the surgeon's preference or
the time required to accomplish complete repair, particularly
in the presence of a ventricular septal defect or other anomalies
such as coarctation of the aorta or a hypoplastic or interrupted
aortic arch. In a patient with D-transposition of the great
arteries and an intact ventricular septum, the operation is
preferably performed with the patient under either total circulatory
arrest or continuous low-flow (50 ml/kg/min) hypothermic perfusion,
limiting circulatory arrest time to the few minutes necessary
to close the atrial septal defect. In the presence of a ventricular
septal defect or other complex associated lesions, two periods
of deep hypothermic circulatory arrest are used, interposing
10 to 15 minutes of hypothermic reperfusion is between them,
or the arterial switch itself may be performed under continuous
low-flow cardiopulmonary bypass, with profound hypothermic circulatory
arrest for closure of the ventricular septal defect and other
procedures such as repair of an interrupted aortic arch.
Stage I: Preparation
o Aprotinin, solumedrol (30 mg/kg), Regitine
(0.1 mg/kg), and prophylactic antibiotics are given preoperatively.
o The sternum is opened, the patient heparinized, and a large
segment of pericardium is harvested and prepared with 0.6% glutaraldehyde.
o The coronary arteries and great vessels are inspected.
o The arterial duct is dissected free, as are the left and right
pulmonary arteries, including the first pulmonary artery branches
in the hilum of each lung. The right pulmonary artery can be
dissected prior to bypass, and the left dissected while on bypass.
o The ascending aorta is cannulated as far distally as possible
to allow adequate length for the aortic anastomosis. A single
venous cannula is placed within the right atrium. The left ventricle
is vented with a catheter placed in the right superior pulmonary
vein.
Stage II: Cardiopulmonary
o Cardiopulmonary bypass is begun, and the
patient cooled for a minimum of 20 minutes to 20°C rectal
temperature.
o The arterial duct is doubly ligated and divided, and the branch
pulmonary arteries are completely mobilized.
o The site of aortic transection is marked before the cross
clamp is applied. This is just distal to the pulmonary artery
bifurcation, as best judged by the take-off of the left pulmonary
artery.
o At 20°C rectal temperature, the distal ascending aorta
is clamped, and cold blood cardioplegia is delivered into the
proximal ascending aorta.
o The aorta is divided at the previously marked site, and the
main pulmonary artery is divided just proximal to its bifurcation.
stage, adhesions do not usually present a problem. In the unusual
situation in which the origin of the left coronary artery cannot
be visualized after the banding, the arterial switch operation
is deferred (for about 12 months) at which time clear delineation
of the coronary anatomy can be made by coronary arteriography
and/or magnetic resonance imaging.
o The aortic and pulmonary valves are careftilly inspected,
as is the presence of left ventricular outflow tract obstruction.
o The Lecompte maneuver is performed, and the pulmonary artery
is held in position anterior to the ascending aorta by moving
the aortic cross clamp.
o The anterior commissure of the neoaorta is marked with a silk
suture. Alternatively, the exact positions of the implantation
sites are identified by juxtaposing the explanted coronary arteries
or by placing marking sutures before cardiopulmonary bypass,
when the aortic and pulmonary roots are distended.
Stage III: Coronary Transfer
o The ostium, the initial course of the
left and right coronary arteries, and the presence of infundibular
branches are identified.
o The coronary ostia are excised along with a large segment
of surrounding aortic wall, extending the incision well into
the base of the sinus of Valsalva.
o The proximal coronary arteries are mobilized sufficiently
to avoid tension or distortion. Infundibular branches are very
rarely sacrificed.
o The distal aorta is anastomosed to the proximal neoaorta with
a continuous 6-0 Prolene or Maxon.
o The coronary implantation sites are prepared by making a neoaortotomy
into the left and right anterior aspects of the neoaorta while
the aortic cross-clamp is temporarily removed, angling the incisions
from posterior to anterior, and using the commissural marking
stitch as a guide.
o The coronary ostia are transferred by sewing the coronary
flaps to these incisions with a continuous 7-0 Maxon suture.
o When the circumflex coronary artery arises
from the right coronary artery, the site of right coronary implantation
must be placed either higher than usual on the proximal neoaorta
or, occasionally, above the suture line on the distal ascending
aorta to avoid distortion of the circumflex artery.
o Adequate mobilization of the right coronary artery is frequently
necessary to avoid distortion of the circumflex coronary artery.
If the two coronary arteries originate from the same sinus,
they can often be included in the same aortic flap (provided
there is not an intramural course for one of the coronaries).
o If the coronary ostia are located closely adjacent (paracommissural)
to the posterior commissure, excision of a segment of the posterior
commissure of the native aortic valve (neopulmonary valve) is
often necessary; the resultant neopulmonary regurgitation is
generally mild and well tolerated.
Stage IV: Circulatory Arrest
o At this point, the pump is turned off
and the venous cannula removed. The atrial communication is
closed through a right atriotomy, which, as a rule, can be accomplished
by suture closure after balloon septostomy, as there is usually
no tissue deficiency.
Stage V: Right Ventricular Outflow Reconstruction
o The atriotomy is closed, and the aortic
and venous cannula replaced.
o Cardiopulmonary bypass is resumed and the aortic cross-clamp
removed.
o The left ventricular vent is turned on.o The aortic and pulmonary
valves are careftilly inspected, as is the presence of left
ventricular outflow tract obstruction.
o The Lecompte maneuver is performed, and the pulmonary artery
is held in position anterior to the ascending aorta by moving
the aortic cross clamp.
o The anterior commissure of the neoaorta is marked with a silk
suture. Alternatively, the exact positions of the implantation
sites are identified by juxtaposing the explanted coronary arteries
or by placing marking sutures before cardiopulmonary bypass,
when the aortic and pulmonary roots are distended.
Stage III: Coronary Transfer
.The ostium, the initial course of the left
and right coronary arteries, and the presence of infundibular
branches are identified.
.The coronary ostia are excised along with a large segment of
surrounding aortic wall, extending the incision well into the
base of the sinus of Valsalva.
.The proximal coronary arteries are mobilized sufficiently to
avoid tension or distortion. Infundibular branches are very
rarely sacrificed.
.The distal aorta is anastomosed to the proximal neoaorta with
a continuous 6-0 Prolene or Maxon.
.The coronary implantation sites are prepared by making a neoaortotomy
into the left and right anterior aspects of the neoaorta while
the aortic cross-clamp is temporarily removed, angling the incisions
from posterior to anterior, and using the commissural marking
stitch as a guide.
.The coronary ostia are transferred by sewing the coronary flaps
to these incisions with a continuous 7-0 Maxon suture.
. When the circumflex coronary artery arises
from the right coronary artery, the site of right coronary implantation
must be placed either higher than usual on the proximal neoaorta
or, occasionally, above the suture line on the distal ascending
aorta to avoid distortion of the circumflex artery.
o Adequate mobilization of the right coronary artery is frequently
necessary to avoid distortion of the circumflex coronary artery.
If the two coronary arteries originate from the same sinus,
they can often be included in the same aortic flap (provided
there is not an intramural course for one of the coronaries).
o If the coronary ostia are located closely adjacent (paracommissural)
to the posterior commissure, excision of a segment of the posterior
commissure of the native aortic valve (neopulmonary valve) is
often necessary; the resultant neopulmonary regurgitation is
generally mild and well tolerated.
Stage IV: Circulatory Arrest
.At this point, the pump is turned off and
the venous cannula removed. The atrial communication is closed
through a right atriotomy, which, as a rule, can be accomplished
by suture closure after balloon septostomy, as there is usually
no tissue deficiency.
Stage V: Right Ventricular Outflow Reconstruction
.The atriotomy is closed, and the aortic
and venous cannula replaced.
.Cardiopulmonary bypass is resumed and the aortic cross-clamp
removed.
.The left ventricular vent is turned on.
.Full-flow and rewarming are begun. An additional dose of Regitine
0.1 mg/kg is given in the pump.
.The coronary explantation sites in the neopulmonary artery
are then filled, using a single, long, inverted bifurcated patch
of 0.6% glutaraldehyde-pretreated, autologous pericardium. An
incision is made into the pericardium to fit into the posterior
commissure, and the free pericardial edge is sutured to the
area of the aorta (neopulmonary artery) corresponding to the
explanted coronary flaps, using a continuous 6-0 suture. When
the anterior remnant of the aortic wall is reached, the pericardium,
at this point cylindrically shaped, is tailored to bridge the
distance between the proximal neopulmonary artery and the distal
pulmonary artery without tension. Discrepancies in caliber between
the proximal neopulmonary artery and the distal pulmonary artery
are reconciled with this pericardial extension.
o Alternatively, two separate pericardial patches can be used,
one for the site of each coronary donor.
o The relationship of the great vessels will require other certain
modifications. With side-by-side great vessels, for example,
a Lecompte maneuver is not always performed, and the central
stoma in the transverse pulmonary artery is moved to the right
pulmonary artery.
o The proximal neopulmonary artery is anastomosed to the bifurcation
of the native pulmonary artery. Some authors prefer to place
the bifurcated pericardial patch as the first maneuver after
removing the coronary arteries from the aorta and before coronary
reimplantation in some cases.
Stage IV: Completing the Operation
. Pleural tubes, along with left atrial,
right atrial, and pulmonary artery lines are placed and secured,
as are atrial and ventricular temporary pacemaker leads. Ventilation
is resumed, and the patient is weaned off cardiopulmonary bypass.
Neuromuscular blockade, continuous fentanyl sedation, mechanical
ventilation, and moderate inotropic support are customarily
maintained during the first 12 to 18 hours or until hemodynamic
stability is achieved.
Rapid Two-Stage Repair of d-TGA
with intact ventricular septum
The First Stage
Through either a right thoracotomy or a
midline sternotomy, a 3.5- or 4-mm polytetrafluoroethylene (GoreTex)
graft is used to connect the right subclavian artery to the
right pulmonary artery. Subsequently, and after minimal dissection,
a Dacron-reinforced Silastic band is tightened around the main
pulmonary artery to achieve a left ventricular pressure that
is approximately 75% of systemic pressure. The pericardium is
then loosely closed after thoroughly irrigating the pericardial
space with heparinized saline to flush out any residual blood
or fibrin clots.
The Second Stage
The only modification required relative
to the standard operative approach for the arterial switch operation
is to first divide and oversew the modified Blalock-Taussig
shunt, and to remove the pulmonary artery band. Because the
second stage is carried out an average of 7 days after the first
stage,adhesions do not usually present a problem. In the unusual
situation in which the origin of the left coronary origin cannot
be visualized after the banding,the arterial switch operation
is deferred( for about 12 months) at which time clear delineation
of the coronary anatomy can be made by coronary arteriography
and /or magnetic resonance imaging.
Repair of d-TGA with ventricular septal
defect and left ventricular outflow tract obstruction
The conventional treatment for neonates
and infants with D-transposition of the great arteries, a ventricular
septal defect, and hemodynamically significant left ventricular
outflow tract obstruction has been an initial Blalock-Taussig
shunt. However, either direct relief of the obstruction is attempted,
accompanied by ventricular septal defect closure and an arterial
switch operation, or, in the case of a long-segment hypoplastic
left ventricular outflow tract obstruction or valvar pulmonary
stenosis, a Rastelli operation using a cryo-preserved valved
aortic or pulmonary homograft is performed (particularly in
the neonate or young infant in whom the severe cyanosis is due
in part to poor mixing, in spite of the possibility of adequate
or even over circulation of the pulmonary vascular bed).
Arterial switch operation, ventricular
septal defect closure, and direct resection of LVOTO
The occasional discrete subpulmonary membrane
or excrescence of endocardial cushion tissue is easily resected
through the posterior (pulmonary) semilunar valve. More common,
and surgically more demanding, is left ventricular outflow tract
obstruction caused by a posteriorly deviated outlet septum.
Once the ascending aorta and main pulmonary
artery are divided in the course of an arterial switch operation,
the obstructing muscle is more safely exposed through the pulmonary
semilunar valve. Although exposure of the outlet septum is often
easier through the anterior (aortic) semilunar valve, in patients
with D-transposition of the great arteries, incision and excision
of the outlet septum via the aortic valve run the risk of damaging
the pulmonary valve, because the pulmonary semilunar valve originates
at a lower level than the aortic semilunar valve. Therefore,
the trans-pulmonary approach allows a more aggressive excision
of the posteriorly deviated outlet septum, at the same time
leaving sufficient muscle to anchor the ventricular septal patch.
It helps to engage the outlet septum with a skin hook and to
deliver it further into the left ventricular outflow tract before
excising the muscle mass.
Rastelli operation for d-TGA
Often, in the case of a long, hypoplastic
left ventricular outflow tract, resection is not feasible. In
such cases a Rastelli operation is preferred to a palliative
shunt operation, regardless of the patient's age. After opening
the chest through a midline sternotomy, an appropriate-size
valved homograft (aortic or pulmonary) is selected, usually
varying in size from 9 mm for a neonate to 14 mm for an older
infant. In addition, a patch of pericardium is harvested and
pretreated with 0.6% glutaraldehyde for later use to augment
the anastomosis from the right ventricle to the homograft. Depending
on the age and size of the child, either circulatory arrest
or cardiopulmonary bypass with low-flow hypothermic perfusion
is used. The main pulmonary artery commonly lies posterior and
to the left of the ascending aorta, and its branches are dissected
and the ligamentum arteriosum divided. If continuous cardiopulmonary
bypass is used, the aorta is cross clamped at 25°C, and
cold cardioplegia is injected. A vertical right ventriculotomy
is then made to expose the aortic valve, the ventricular septal
defect and the tricuspid valve. Unless the malaligned septal
defect is larger than the diameter of the aortic valve, it is
enlarged by making two incisions (at 2 o'clock and 4 o'clock)
into the anterosuperior limb of the septal band. The intervening
muscle is excised. This maneuver is important to achieve an
unobstructed pathway between the left ventricle and the aorta.
Interrupted horizontal mattress sutures, reinforced with Teflon
pledgets, are placed first along the posteroanterior rim of
the defect in a manner similar to the technique used for closure
of a malaligned ventricular septal defect in tetralogy of Fallot.
Additional interrupted stitches are then placed within the remaining
circumference of the pathway from the left ventricle to the
aortic valve. A baffle is then tailored from a tubular Dacron
conduit (retaining approximately 50% of the circumference of
the conduit), measuring the distance from the enlarged ventricular
septal defect to the aortic valve rim. The sutures placed along
the anterior border of the ventricular septal defect and the
posteroanterior aspect of the ventricular defect are first threaded
through the Dacron baffle and then tied in place. This partial
fixation of the baffle offers the opportunity for adjustments
in its length or width. The remainder of the sutures are then
passed through the Dacron patch and tied. The Dacron baffle
should contribute approximately 50% to the circumference of
the pathway from the left ventricle to the ascending aorta,
the remainder being composed of the patient's own tissue. Next,
either the aortic or the pulmonary valve homograft is prepared
to cover the distance between the distal main and proximal left
pulmonary artery and the right ventriculotomy. To avoid extrinsic
compression of the homograft, the conduit is aligned along the
left heart border; the left mediastinal pleura is opened to
gain additional space for the conduit. After doubly ligating
the main pulmonary artery proximally, the distal conduit-to-pulmonary
artery anastomosis is fashioned with a 6-0 continuous suture.
At this point the aortic cross clamp is removed. During rewarming,
the anastomosis between the right ventricle and the homograft
is begun at the most distal part of the ventriculotomy incision
and is extended to include approximately 50% of the circumference
of the proximal homograft stoma. At that point, the glutaraldehyde-preserved
pericardial patch is sewn to the remaining part of the right
ventriculotomy and to the free edge of the proximal homograft.
This technique eliminates distortion of the anastomosis and
ensures unobstructed flow through the homograft. After the air
is vented and effective cardiac action has resumed, the infant
is weaned from cardiopulmonary bypass. Catheters are routinely
placed in the left and right atria and also in the trans-homograft
pulmonary artery for postoperative monitoring.
The Arterial Switch Operation for Double
Outlet Right Ventricle
An arterial switch operation is indicated
for double outlet right ventricle which is at the dtransposition
end of the spectrum - when there is little to no pulmonary or
subpulmonary stenosis. It may be possible to resect muscular
or fibrous tissue from the subpulmonary region as long as there
is no important straddling mitral valve chordae. Similarly,
a bicuspid pulmonary valve should not be considered an absolute
contraindication to an arterial switch, particularly since both
an atrial inversion procedure or a complex intraventricular
repair with a conduit may result in a lesser quality of life
The general principles of the arterial switch
operation for double outlet right ventricle are identical to
those employed in the operation for d-transposition. The procedure
is generally performed using low-flow hypothermic bypass for
the extracardiac portion of the procedure while the intracardiac
steps (i.e., closure of the atrial and ventricular septal defects)
are conveniently performed during a period of circulatory arrest.
Division of the great arteries is followed by inspection of
the pulmonary valve and left ventricular outflow tract to ensure
that there is no important outflow tract obstruction that might
increase the risks from an arterial switch. Coronary mobilization
and transfer are performed, followed by the aortic anastomosis.
It is preferable not to undertake closure of the intracardiac
communications before these steps are taken, as they will allow
venting of left heart
The Arterial Switch Operation for Double
Outlet Right Ventricle
An arterial switch operation is indicated
for double outlet right ventricle which is at the dtransposition
end of the spectrum - when there is little to no pulmonary or
subpulmonary stenosis. It may be possible to resect muscular
or fibrous tissue from the subpulmonary region as long as there
is no important straddling mitral valve chordae. Similarly,
a bicuspid pulmonary valve should not be considered an absolute
contraindication to an arterial switch, particularly since both
an atrial inversion procedure or a complex intraventricular
repair with a conduit may result in a lesser quality of life
The general principles of the arterial switch
operation for double outlet right ventricle are identical to
those employed in the operation for d-transposition. The procedure
is generally performed using low-flow hypothermic bypass for
the extracardiac portion of the procedure while the intracardiac
steps (i.e., closure of the atrial and ventricular septal defects)
are conveniently performed during a period of circulatory arrest.
Division of the great arteries is followed by inspection of
the pulmonary valve and left ventricular outflow tract to ensure
that there is no important outflow tract obstruction that might
increase the risks from an arterial switch. Coronary mobilization
and transfer are performed, followed by the aortic anastomosis.
It is preferable not to undertake closure of the intracardiac
communications before these steps are taken, as they will allow
venting of left heart return to the single right atrial cannula.
The single cannula is preferred to two caval cannulae for the
same reason, as well as for the improved exposure provided by
one cannula as compared with two.
The ventricular septal defect may be approached
through the anterior semilunar valve, through the right atrium,
or through a right ventriculotomy, as determined by the specific
anatomic situation. Often there is some element of subaortic
narrowing, so a right ventricular infundibular incision serves
a dual purpose: access for closure of the ventricular septal
defect and access for placement of an infundibular outflow patch
to relieve outflow tract obstruction Approach through the semilunar
valve or ventriculotomy often allows continuation of bypass
throughout closure of the ventricular septal defect. The atrial
septal defect is closed through a short, low right atriotomy,
with the left heart filled with saline to exclude air before
tying the suture.
With bypass re-established, the aortic cross
clamp is released. Perfusion of all areas of the myocardium
is checked. A single large pericardial patch is used to reconstruct
the coronary donor areas, although to obtain optimal exposure,
this step may be performed before the intracardiac steps. It
is important that the pericardial patch actually supplement
the neopulmonary artery (i.e., the patch needs to be quite a
bit larger than the excised coronary buttons, because the aorta
is frequently somewhat smaller than the pulmonary artery, particularly
if there is a long and somewhat narrow subaortic conus). The
pulmonary anastomosis is fashioned, and the patient is weaned
from bypass. Specific variations of the arterial switch operation
for double outlet right ventricle include:
Coronary patterns.
Unusual coronary patterns are much more
common with side-by-side great arteries than in standard transposition
with anteroposterior great arteries. A common pattern is an
anterior origin of the right and left anterior descending coronary
arteries from a single ostium, with the circumflex originating
from a posterior facing sinus. Extensive mobilization of the
right coronary is necessary to prevent tethering of the anterior
coronary, which must be transferred directly away from the line
of the right coronary. Infundibular and right ventricular free
wall branches of the right and anterior descending coronaries
should be extensively mobilized from their epicardial beds to
prevent tension on the arteries and on the anastomosis. On occasion,
an autologous pericardial tube extension of the coronary artery
can be used to avoid excessive tension. Excessive tension will
be manifested by persistent bleeding from the coronary anastomosis
and early or late coronary insufficiency. Another common coronary
pattern with side-by-side great arteries is origin of the right
and circumflex coronaries from the posterior sinus, with the
left anterior descending artery originating from the anterior-facing
sinus. It is important to guard against compression of the anteriorly
transferred coronary by the posterior wall of the main pulmonary
artery.
Closure of the ventricular septal defect.
Exposure of the ventricular septal defect
associated with double outlet right ventricle may present special
difficulties. The defect may be quite leftward and anterior
in what almost appears, from the surgeon's perspective, to be
a separate, leftward, blind-ending infundibular recess Exposure
through the anterior semilunar valve and right atrium is particularly
difficult, and even through a right ventriculotomy it may not
be easily seen. Although exposure may be achieved through the
original pulmonary valve, this is usually not recommended because
of the risk of damage to the conduction system and the neoaortic
valve. An additional complication to ventricular septal defect
closure in this setting is the tendency for the very leftward
ventricular septal defect to extend into the anterior trabeculated
septum - that is, there appears to be no clear leftward and
anterior margin to the defect. By taking large bites with pledgetted
sutures, the size of any residual ventricular septal defect
can be minimized. Catheter-delivered devices have been useful
for ultimate closure of residual ventricular septal defects
in this area.
Multiple ventricular septal defects.
Surgical closure of multiple muscular ventricular
septal defect as well as large subpulmonary ventricular septal
defect may be difficult and may consume an excessive amount
of circulatory arrest time. One approach to this problem is
intraoperative delivery o a double-clamshell device. After division
of the two great vessels, an excellent view is obtained of both
sides of the ventricular septum. The sheath loaded with the
device is introduced through the right atrium and tricuspid
valve into the right ventricle A right-angled instrument is
passed through the original pulmonary valve into the left ventricle
through the ventricular septal defect, and into the right ventricle,
where it grasps the delivery pod The pod is drawn into the left
ventricle, and the lei ventricular arms are released under direct
vision The pod is then carefully pulled back into the right
ventricle, and, viewing through the original aortic valve into
the right ventricular arms are released. If necessary, multiple
devices may be placed. Although this system has worked well
for children weighing more than 4 to 5 kg, the delivery pod
requires further modification for neonates and infants weighing
less than 4 kg.
Pulmonary artery anastomosis.
Although the Lecompte maneuver is uniformly
useful for patients with standard transposition in which the
great arteries are positioned antero-posteriorly (or relatively
close to this), for side-by-side great arteries judgment is
required in deciding whether translocation of the right pulmonary
artery anterior to the aorta will be useful in decreasing tension
on the right pulmonary artery. In general, if the aorta is the
slightest bit anterior to the pulmonary artery a Lecompte maneuver
should be performed. Another consideration in this decision,
other than just the tension on the right pulmonary artery, is
the relationship of the transferred coronary arteries to the
pulmonary artery. Care must be taken to ensure that there is
no compression of the coronary arteries. A useful maneuver to
minimize the risk of coronary compression, as well as to decrease
the tension on the pulmonary artery anastomosis, is to shift
the anastomosis somewhat from the original distal divided main
pulmonary artery into the right pulmonary artery. The leftward
end of the main pulmonary artery is closed (usually by direct
suture, although the pericardial patch used to fill the coronary
donor areas may be extended here), and the orifice is extended
into the right pulmonary artery. In other respects the anastomosis
is performed in the usual fashion. This maneuver has the effect
of shifting the main pulmonary artery rightward so that it will
not lie anterior to the aorta where it would likely cause compression
of the anteriorly transferred coronary artery.
Repair of subaortic stenosis and arch
anomalies.
The long subaortic conus associated with
double outlet right ventricle toward the D-transposition end
of the spectrum may cause some degree of subaortic stenosis.
Not surprisingly, aortic arch hypoplasia and coarctation often
accompany such subaortic stenosis. There is likely to be considerable
disparity between the diameters of the great vessels. During
the preliminary phase of the arterial switch procedure tourniquets
should be loosely applied around the head vessels. The coronary
transfer should be undertaken in the usual fashion, using low-flow
bypass. The circulation is then arrested, the tourniquets are
tightened, and the aortic cross clamp is removed. An incision
is made along the lesser curve of the ascending aorta and arch,
extending across the coarctation. A long patch of pericardium
is sutured into this aortotomy, which serves to minimize the
disparity between the proximal neoaorta and the distal ascending
aorta. The aortic cross clamp is reapplied, and bypass may be
recommended. The remainder of the procedure is undertaken as
described previously. Coarctation repair and pulmonary artery
banding are not favored as preliminary maneuvers.
http://www.pediheart.org/practitioners/operations/ASO.html
d)
Eisenmenger's Syndrome
This
consist of a large left (L) to right (R) shunt, which causes
severe pulmonary (lung) vascular disease and high blood pressure
(in the lungs) with resulting reversal of the direction of shunting
(figure 23j).
This shunting with increase pressure causes the lung arteries
to narrow due to thickening of their walls (especially the middle
wall, called tunica media, (see figure 23j)
and cause obstruction. Initially the changes may be reversible,
but ultimately they become irreversible due to inflammation
of the arteries. Hence, much of the lung arteries are occluded,
leading to increase pulmonary blood vessel resistance. Ultimately
the resistance in the lungs may exceed the resistance in the
arteries of the rest of the body, which leads to a reversal
of flow from left-to-right to right-to-left shunt.
The
reversal of the shunt leads to cyanosis, as well as shortness
of breath, coughing up blood, reduced exercise tolerance, syncope
(fainting), palpitations, and atrial fibrillation (see figures
15A,
15B).
Brain events such paradoxical embolus, thrombosis (stroke) and
hemorrhage may occur. Heart failure suggest a poor prognosis,
and sudden death is possible.
Digital
swelling (clubbing) may occur. Heart murmurs may occur.
EKG
may show RVH and atrial arrhythmias (see figures 2,
3a,
5a,
5b,
14,
15a,
15b).
Echocardiogram
shows RV pressure overload, pulmonary high blood pressure, and
the underlying heart defect. Using intravenous contrast injections
along with echocardiogram will visualize the intracardiac defect.
Heart catherterization is necessary to assess the lung hypertension
and the size of the defect.
Rate
of survival is 80% 10 years after diagnosis, 77% at 15 years,
and 42% at 25 years. Death is usually sudden, presumably due
to arrhythmias, but some die of the above mentioned complications.
Lung
transplantation with repair of the cardiac or combined heart-lung
transplantation is an option for patients with a poor prognosis
(failing to respond to medical therapy).
Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
e)
Hypoplastic Left Heart Syndrome
(Article by P.Syamasundar Rao,MD
and Others, E-Medicine from WebMD, August 15,2006,Pages 1-24).
Hypoplastic Left Heart Syndrome
Background:
Hypoplastic left heart syndrome (HLHS) describes
a spectrum of cardiac abnormalities characterized by marked
hypoplasia of the left ventricle and ascending aorta(see figures
and video clips below). The aortic and mitral valves are atretic,
hypoplastic, or stenotic (figures 44g-1, 44g-2 and figure 23a).
The ventricular septum is usually intact. A large patent ductus
arteriosus supplies blood to the systemic circulation. Systemic
desaturation may be present because of complete mixing of pulmonary
and systemic venous blood in the right atrium via an atrial
septal defect or patent foramen ovale. Coarctation of the aorta
commonly coexists (figure 23a).
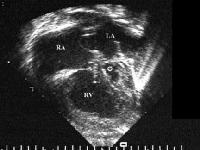
Figure 44g-3:
HLHS 4 chamber echocardiographicview slide showing the small
left ventricle (star), the large right atrium, right ventricle
and the left atrium.
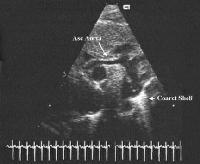
Figure 44g-4:
Still frame long axis view of aortic arch showing the small
ascending aorta and arch serving only to deliver blood in a
retrograde fashion to the coronary arteries, an echobright coarctation
shelf is seen at the insertion of the ductus arteriosus.
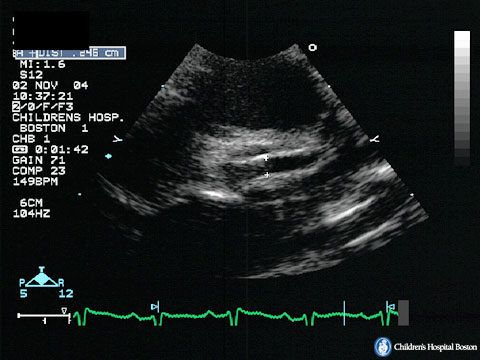
Figure 44g-4:
Echocardiograhic view of small undeveloped ascending aorta in
HLH.
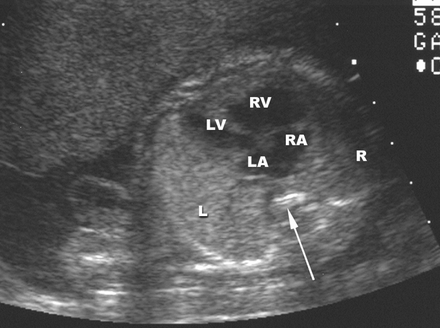
Figure 44g-5:
Hypoplastic left heart syndrome in a fetus with a cephalic presentaton,
Transabdominal US image(four-chamber view) shows that the left
ventricle is small relative to the right ventricle and the left
atrium is small relative to the right atrium. Arrow= spine.
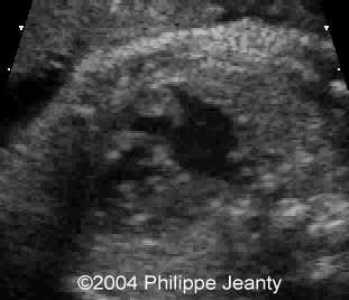
Figure 44g-6:
Large right atrium and ventricle compared to the left side.
(From Dr. Philippe Jeanty and others, 2004).
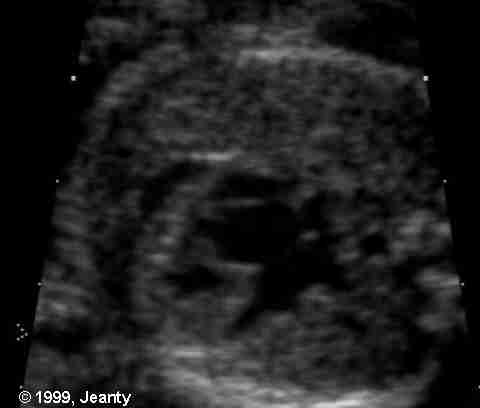
Figure 44g-7:
HLHsyndrome. From Dr. Philippe Jeanty,
1999.
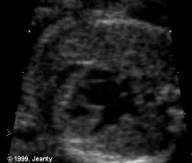
Figure 44g-8:
HLH with VSD change. From Dr. Philippe
Jeanty, 1999.
Hypoplastic left heart syndrome is a uniformly
lethal cardiac abnormality if not surgically corrected. In 1979,
Norwood performed the first successful surgical palliation on
a neonate. Currently, this approach consists of a series of
3 operations: the Norwood procedure (stage I), the hemi-Fontan
or bidirectional Glenn procedure (stage II), and the Fontan
procedure (stage III). Orthotopic heart transplantation provides
an alternative therapy, with results similar to those of the
staged surgical palliation. Currently, the survival rate of
infants treated with these surgical approaches is similar to
that of infants with other complex forms of congenital heart
disease in which a 2-ventricle repair is not possible.
Pathophysiology:
The newborn infant with hypoplastic left heart
syndrome has a complex cardiovascular physiology. Fully saturated
pulmonary venous blood returning to the left atrium cannot flow
into the left ventricle because of atresia, hypoplasia, or stenosis
of the mitral valve. Therefore, pulmonary venous blood must
cross the atrial septum and mix with desaturated systemic venous
blood in the right atrium. The right ventricle then must pump
this mixed blood to both the pulmonary and the systemic circulations
that are connected in parallel, rather than in series, by the
ductus arteriosus. Blood exiting the right ventricle may flow
(1) to the lungs via the branch pulmonary arteries or (2) to
the body via the ductus arteriosus and descending aorta. The
amount of blood that flows into each circulation is based on
the resistance in each circuit.
Blood flow is inversely proportional to resistance
(Ohm law); that is, when resistance in blood vessels decreases,
blood flow through these vessels increases. Following birth,
pulmonary vascular resistance decreases, which allows a higher
percentage of the fixed right ventricular output to go to the
lungs instead of the body. Although increased pulmonary blood
flow results in higher oxygen saturation, systemic blood flow
is decreased. Perfusion becomes poor, and metabolic acidosis
and oliguria may develop. Coronary artery and cerebral perfusion
also are dependent on systemic blood flow through the ductus
arteriosus. Therefore, increased pulmonary blood flow results
in decreased flow to the coronary arteries and brain, with a
risk of myocardial or cerebral ischemia.
Alternatively, if pulmonary vascular resistance
is significantly higher than systemic vascular resistance, systemic
blood flow is increased at the expense of pulmonary blood flow.
This may result in profound hypoxemia. A careful delicate balance
between pulmonary and systemic vascular resistance ensures adequate
oxygenation and tissue perfusion.
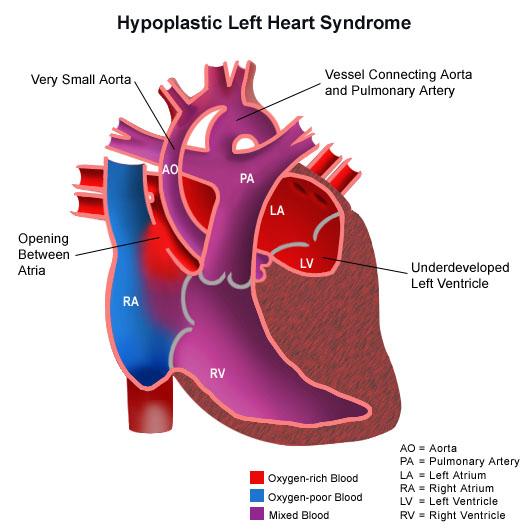
Hypoplastic left heart syndrome with
color to show various degrees of oxgenated blood in various
chambers of heart
Most patients with hypoplastic left heart
syndrome also demonstrate coarctation of the aorta (figure 23a).
This can be significant enough to interfere with retrograde
flow to the proximal aorta.
Frequency:
* In the US: Incidence of hypoplastic left
heart syndrome is 0.16-0.36 per 1000 live births. Hypoplastic
left heart syndrome accounts for 7-9% of all congenital heart
disease diagnosed in the first year of life. Before surgical
treatment was available, hypoplastic left heart syndrome was
responsible for 25% of cardiac deaths in the neonatal period.
The rate of occurrence is increased in patients with Turner,
Noonan, Smith-Lemli-Opitz, or Holt-Oram syndrome. Certain chromosomal
duplications, translocations, and deletions also are associated
with hypoplastic left heart syndrome.
* Internationally: Frequency is similar to
that in the United States.
Mortality/Morbidity:
* Without surgery, hypoplastic left
heart syndrome is uniformly fatal usually within the first 2
weeks of life. Survival for a longer period occurs rarely and
only with persistence of the ductus arteriosus.
* Following the Norwood procedure (stage
I), overall success (survival to hospital discharge) is approximately
75%. Success rates are higher (85%) in patients with low preoperative
risk and lower (45%) in patients with important risk factors.
Some centers have reported stage I survival rates in excess
of 90%. This appears to be related, in part, to institutional
surgical volume. The overall success following the hemi-Fontan
procedure (stage II) approaches 95%. Success after completing
the Fontan procedure (stage III) approaches 90%. Orthotopic
heart transplantation results in early and long-term success
similar to that of staged reconstruction. Among low-risk patients
who undergo staged reconstruction or transplantation, actuarial
survival at 5 years is approximately 70%.
* Most studies report neurodevelopmental
disabilities in a significant number of patients who survive
either staged surgical reconstruction or cardiac transplantation.
Sex:
Hypoplastic left heart syndrome is more common
in males than in females, with a 55-70% male predominance.
Age:
Hypoplastic left heart syndrome typically
presents within the first 24-48 hours of life. Presentation
occurs as soon as the ductus arteriosus constricts, thereby
decreasing systemic blood flow, producing shock, and, without
intervention, causing death. Infants with pulmonary venous obstruction
(absent or restrictive patent foramen ovale) may present sooner.
Very rarely, an infant with persistence of high pulmonary vascular
resistance and the ductus arteriosus may present later because
of balanced pulmonary and systemic blood flow.
History:
* Although hypoplastic left heart syndrome
can easily be detected on fetal echocardiography (see below
figures and video clips including fetal cardiovascular anatomy
and standard 2D ultrasound examination of the heart, movie-
1, HLH, and ultrasound.zip item), many infants are not identified
prenatally because routine obstetric ultrasound examination
may not concentrate on cardiac anatomy. Pregnancies are typically
uncomplicated, and fetal echocardiography is not indicated routinely.
The fetus grows and develops normally because the fetal circulation
is not altered significantly. Most neonates are born at term
and initially appear normal.
Fetal Echocardiography by Gregory R. DeVore,
M.D. describing cardiovascular anatomy, and standard two dimensional
ultrasound examination of the heart, including some abnormal
conditions.
Video clip
1: Fetal
echocardiogram showing a four chamber view of the heart with
the aorta and pulmonary arteriy visualized.
Video clip 2: HLH:
Ultrasound
video clip: From
Phillipe Jeanty,M.D., PhD, Chiatali Shah,M.D., Crine Jeanty:
Hypoplastic Left Heart Syndrome at www.The fetus.net showing
the smaller left ventricle , the markedly reduced size of the
ascending aorta, the normal pulmonary artery and the normal
right ventricle, the enlarged right atrium and smaller left
artium.
* Occasionally, respiratory symptoms
and profound systemic cyanosis are apparent at birth (2-5% of
cases). In these infants, significant obstruction to pulmonary
venous return (a congenitally small or absent patent foramen
ovale) is usually present.
* As the ductus arteriosus begins to close
normally over the first 24-48 hours of life, symptoms of cyanosis,
tachypnea, respiratory distress, pallor, lethargy, metabolic
acidosis, and oliguria develop. Without intervention to reopen
the ductus arteriosus, death rapidly ensues.
Physical:
* Before the initiation of prostaglandin
E1 infusion to reestablish patency of the ductus arteriosus,
infants exhibit signs of cardiogenic shock, including the following:
o Hypothermia
o Tachycardia
o Respiratory distress
o Central cyanosis and pallor
o Poor peripheral perfusion with
weak pulses in all extremities and in the neck
o Hepatomegaly
* After reestablishment of systemic
blood flow via the ductus arteriosus, signs of shock resolve,
leaving the stable infant with tachycardia, tachypnea, and mild
central cyanosis. If a coarctation of the aorta is present,
arterial pulses in the legs may be more prominent than those
in the arms, particularly the right arm.
* Cardiac examination
o Palpable right ventricular
impulse
o Normal first heart sound
o Loud single second heart sound
o Nonspecific, soft, systolic ejection
murmur at the left sternal border (not always present)
o High-pitched holosystolic murmur
at the lower left sternal border, indicating tricuspid regurgitation
(not always present)
o Diastolic flow rumble over the
precordium, indicating increased right ventricular diastolic
filling (not always present)
Causes:
* The exact cause of hypoplastic left
heart syndrome is unknown. Most likely, the primary abnormality
occurs during aortic and mitral valve development. During cardiac
development, adequate flow of blood through a structure is largely
responsible for the growth of that structure. With little or
no blood flow because of aortic and mitral valve atresia, growth
of the left ventricle does not occur.
* Similarly, growth of the ascending aorta
does not occur because of lack of left ventricular output. The
ascending aorta is perfused in retrograde manner from the ductus
arteriosus functioning only as a common coronary artery.
* Premature closure or absence of the foramen
ovale represents another theoretical cause of hypoplastic left
heart syndrome, as it eliminates fetal blood flow from the inferior
vena cava to the left atrium. Fetal pulmonary blood flow is
not sufficient for normal development of the left atrium, left
ventricle, and ascending aorta.
DIFFERENTIAL DIAGNOSIS:
Aortic Stenosis, Valvar
Atrioventricular Septal Defect, Unbalanced
Cardiac Tumors
Coarctation of the Aorta
Interrupted Aortic Arch
Myocarditis, Viral
Total Anomalous Pulmonary Venous Connection
Other Problems to be Considered:
Associated cardiac abnormalities
Anomalous pulmonary venous connection
Coarctation of the aorta
Complete atrioventricular canal
Coronary artery abnormalities (especially in patients with aortic
atresia and mitral stenosis)
Persistent left superior vena cava
Endocardial fibroelastosis (especially in patients with aortic
atresia and mitral stenosis)
Associated noncardiac abnormalities
Genetic disorders
Significant noncardiac abnormalities
Central nervous system malformation
Diaphragmatic hernia
Necrotizing enterocolitis
Lab Studies:
* Complete blood count
o Measure hemoglobin levels,
because severe neonatal anemia can cause high-output congestive
heart failure (CHF) and cardiogenic shock. The hemoglobin level
is usually normal.
o Obtain a total white blood cell
(WBC) count with differential. Sepsis can cause symptoms of
shock. The WBC count is typically normal.
* Electrolytes
o Electrolyte abnormalities may be
present in infants with poor oral intake secondary to CHF. Use
carbon dioxide to assess acid-base status.
o Electrolyte levels are usually normal. The carbon dioxide
level may be low if a metabolic acidosis is present.
* BUN/creatinine
o Infants with critical illness and
significantly reduced systemic perfusion may show evidence of
renal failure.
o The creatinine may be elevated transiently.
* Liver function tests
o Infants with critical illness and
significantly reduced systemic perfusion and CHF may show evidence
of hepatocellular damage.
o Aspartate aminotransferase (AST) and alanine aminotransferase
(ALT) levels may be elevated transiently.
* Arterial blood gases and lactic acid
o Assessing acid-base status
is paramount, especially to rule out metabolic acidosis. Most
infants have some evidence of metabolic acidosis, which should
be corrected immediately. Elevated levels of serum lactic acid
generally precede a fall in pH, as acidosis develops.
o Assessment of PaO2 and PaCO2 is
important for respiratory management and manipulation of pulmonary
vascular resistance by mechanical ventilation and the addition
of supplemental inhaled nitrogen. The PaO2 is optimally 30-45
mm Hg, and the PaCO2 is ideally 45-50 mm Hg.
* Ultrasound of the head
o An ultrasound of the head is necessary
only if the infant has had a significantly long period in shock
with potentially poor cerebral perfusion.
o Most often, no abnormalities are observed on the ultrasound
scan of the head.
* Karyotype
o Chromosomal analysis is indicated
for infants with dysmorphic features.
o Nearly 25% of infants have chromosomal abnormalities.
Imaging Studies:
* Chest radiograph
o Chest radiographic findings are
not specific for hypoplastic left heart syndrome.
o Cardiomegaly and increased pulmonary venovascular markings
are typically present.
o Marked pulmonary edema may be noted in infants with obstructed
pulmonary venous return.
* Echocardiogram
o The echocardiogram is the
test of choice for diagnosing hypoplastic left heart syndrome.
Two-dimensional imaging clearly shows the hypoplastic left ventricle
and ascending aorta. The right atrium, tricuspid valve, right
ventricle, and main pulmonary artery are larger than usual.
o Other structural abnormalities
should be excluded.
o Doppler and color Doppler imaging
are also important.
o Evaluate tricuspid regurgitation,
a preoperative risk factor for the Norwood procedure, and blood
flow across the atrial septum. Observe retrograde blood flow
from the ductus arteriosus into the transverse aortic arch and
ascending aorta.
o Evaluate the aortic arch and thoracic
aorta for evidence of coarctation.
Other Tests:
* Electrocardiogram
o The electrocardiogram typically
shows sinus tachycardia, right-axis deviation, right atrial
enlargement, and right ventricular hypertrophy with a qR configuration
in the right precordial leads.
o A paucity of left ventricular forces
is noted in the left precordial leads.
Procedures:
* Cardiac catheterization
o Pre?Norwood procedure
+ Routine diagnostic catheterization is not necessary because
2-dimensional and Doppler echocardiography can provide the necessary
anatomic and hemodynamic data.
+ Perform interventional catheterization with blade/balloon
atrial septostomy to relieve pulmonary venous hypertension if
blood flow from left atrium to right atrium is severely restricted
at the atrial septum.
o Pre?hemi-Fontan (stage II)
procedure
+ Perform routine catheterization before the operation to obtain
hemodynamic data and several important angiograms.
+ Calculate pulmonary vascular resistance to ensure the patient's
suitability for the stage II procedure.
+ Perform an angiogram in the right ventricle to show ventricular
function and tricuspid regurgitation.
+ Perform another angiogram in the transverse aortic arch near
the shunt to show pulmonary artery size and distribution and
to rule out recurrent aortic coarctation
? Perform another angiogram in the
transverse aortic arch near the shunt to show pulmonary artery
size and distribution and to rule out recurrent aortic coarctation
or significant aortopulmonary collateral vessels.
? If collateral vessels are found,
they may be occluded with coils at the same catheterization.
o Pre-Fontan (stage III) procedure
+ Accomplish routine catheterization before completing the operation.
+ Calculate pulmonary vascular resistance and perform a right
ventricular angiogram.
+ Delineate pulmonary artery anatomy by performing an angiogram
at the superior vena cava?pulmonary artery anastomosis via an
internal jugular approach.
+ Recurrent coarctation of the aorta and significant collateral
vessels are excluded again.
o Postcatheterization precautions
include hemorrhage, vascular disruption after balloon dilation,
pain, nausea and vomiting, and arterial or venous obstruction
from thrombosis or spasm.
TREATMENT:
Medical Care:
* Successful preoperative management
depends on providing adequate systemic blood flow while limiting
pulmonary overcirculation.
* Open the ductus arteriosus
o Blood flow to the systemic
circulation (coronary arteries, brain, liver, kidneys) is dependent
on flow through the ductus arteriosus. If a diagnosis is suspected,
start prostaglandin E1 infusion immediately to establish ductal
patency and ensure adequate systemic perfusion.
o If the diagnosis is made prenatally or when the infant is
relatively asymptomatic, a smaller dose of prostaglandin E1
may be sufficient to keep the ductus arteriosus patent while
limiting its side effects.
o A larger dose of prostaglandin E1 is often required to reopen
the ductus arteriosus if an infant has cardiovascular collapse
and shock due to ductal closure.
o Ideally, prostaglandin E1 is administered centrally via an
umbilical venous catheter.
* Correct metabolic acidosis
o Metabolic acidosis indicates inadequate
cardiac output to meet the metabolic demands of the body. Acidosis
adversely affects the myocardium.
o Correction of metabolic acidosis with sodium bicarbonate infusion
is essential in early management. This therapy is futile if
the ductus arteriosus remains constricted.
* Manipulate pulmonary vascular resistance
o The pulmonary vascular resistance
of a newborn is slightly less than the systemic vascular resistance
and begins to fall soon after birth. In the patient with hypoplastic
left heart syndrome, decreased pulmonary vascular resistance
causes increased pulmonary blood flow and an undesirable obligatory
decrease in systemic blood flow. Increased alveolar oxygen decreases
pulmonary vascular resistance, leading to increased pulmonary
blood flow. Therefore, most infants should remain in room air
with acceptable oxygen saturation (pulse oximeter) in the low
70s. An exceptional circumstance would be in the infant with
severe hypoxemia caused by pulmonary venous hypertension.
o Achieving a slightly higher PaCO2, in the range of 45-50 mm
Hg, can increase pulmonary vascular resistance. This can be
accomplished by intubation, sedation, mechanical hypoventilation,
or the addition of nitrogen or carbon dioxide to the infant's
inspired gas via the endotracheal tube or hood. It is preferable
not to intubate these infants.
o Serial blood gas analysis is necessary. Initially, an umbilical
arterial catheter is useful to obtain frequent blood samples.
* Inotropes
o Inotropic support is indicated
only in severely ill neonates with concurrent sepsis or profound
cardiogenic shock and acidosis.
o The administration of inotropes can adversely affect the balance
between pulmonary and systemic vascular resistance.
o If needed, wean from inotropic support as soon as the infant
is clinically stable.
* Diuretics
o Consider diuretics to manage
pulmonary overcirculation before surgery.
o Agents commonly used include furosemide and spironolactone.
* Antibiotics
o Antibiotics are indicated if
the infant is at risk for antepartum infection.
o Discontinue antibiotics after obtaining negative blood cultures.
Surgical Care:
* The goal of surgical reconstruction is
to eventually separate the pulmonary and systemic circulations
by completing a Fontan operation. The right ventricle remains
the systemic ventricle while blood flows to the lungs passively.
This ultimate reconstruction is accomplished in 3 stages.
o Norwood procedure (stage I)
+ This procedure is usually performed
during the first weeks of life, after the infant has been stabilized
in the neonatal intensive care unit (ICU). The goals of the
procedure are (1) to establish reliable systemic circulation
in the absence of the ductus arteriosus and (2) to provide enough
pulmonary blood flow for adequate oxygenation, while simultaneously
protecting the pulmonary vascular bed in preparation for stages
II and III.
+ The Norwood procedure includes (1) performing an atrial septectomy
to provide unrestricted blood flow across the atrial septum,
(2) ligating the ductus arteriosus, (3) creating an anastomosis
between the main pulmonary artery and the aorta to provide systemic
blood flow, (4) eliminating coarctation of the aorta, and (5)
placing an aorta?to?pulmonary artery shunt to provide pulmonary
circulation.
+ At hospital discharge, most infants remain on digoxin to augment
cardiac function, on diuretics to help manage right ventricular
volume overload, and on aspirin to prevent thrombosis of the
shunt. If tricuspid regurgitation is present, use afterload
reduction with captopril. Oxygen saturation is typically 70-80%
in room air.
o Hemi-Fontan procedure (stage II)
+ The hemi-Fontan procedure is
performed approximately 6 months after the Norwood procedure.
Before surgery, perform a cardiac catheterization to assess
right ventricular function, pulmonary artery anatomy, and pulmonary
vascular resistance. If results are favorable, schedule elective
surgery.
+ The hemi-Fontan procedure includes creating an anastomosis
between the superior vena cava and the right pulmonary artery,
so that venous return from the upper body can flow directly
into both lungs. The superior vena cava?right atrial junction
is closed with a patch that is removed during the next stage.
Blood from the inferior vena cava continues to drain into the
right atrium. The aorta?to?pulmonary artery shunt that was placed
at stage I is ligated.
+ At discharge, infants usually remain on digoxin, diuretics,
aspirin, and captopril for the reasons mentioned above.
o Fontan procedure (stage III)
+ The Fontan procedure is done
approximately 12 months after the hemi-Fontan procedure. Again,
catheterization is necessary to ensure that the child is a candidate
for surgery.
+ Completion of the Fontan procedure includes directing blood
flow from the inferior vena cava to the pulmonary arteries by
placing a tube within the right atrium. At the conclusion of
the procedure, systemic venous blood returns to the lungs passively
without passing through a ventricle.
+ At discharge, most children remain on digoxin, diuretics,
aspirin, and captopril if necessary. In an uncomplicated case,
most of these medications can be weaned over the 6 months following
the Fontan operation. Some researchers advocate using aspirin
indefinitely.
* Orthotopic cardiac transplantation
o Heart transplantation is
another surgical option. The infant must remain on prostaglandin
E1 infusion to keep the ductus arteriosus patent while waiting
for a donor heart to become available. Approximately 20% of
infants listed for heart transplantation die while waiting for
a suitable donor organ.
o After successful cardiac transplantation,
infants require multiple medications for modulation of the immune
system and prevention of graft rejection. Perform frequent outpatient
surveillance to identify rejection early and prevent lasting
damage to the transplanted heart. Periodic endomyocardial biopsy
usually is performed for more precise monitoring.
Consultations:
* Consult a pediatric cardiologist.
* Consult a pediatric cardiovascular surgeon.
* Consult a genetic specialist if a chromosomal
abnormality is suspected.
Diet:
* Adequate nutrition is important before
and after surgery. Many infants require nasogastric feeding
with increased-calorie breast milk or formula after the Norwood
procedure. However, normal oral feeding is reestablished with
time. Adequate oral iron intake prevents development of iron
deficiency anemia.
* After completion of the Fontan operation,
specific dietary restrictions are not necessary.
Activity:
* Specific activity restrictions are
not imposed on children after completion of the Fontan operation.
In general, encourage children to participate in activities
that they are able to tolerate.
* Studies have shown that these children
may have impaired exercise performance when compared to age-matched
peers. Perform an exercise stress test when the child is old
enough.
* Neurodevelopmental abnormalities occur
often in patients with hypoplastic left heart syndrome.
Medications:
Before the Norwood procedure or cardiac transplantation,
treat infants with prostaglandin E1 infusion, diuretics, inotropes,
and afterload reduction. The medical management after cardiac
transplantation is not discussed in this article.
Drug Category: Prostaglandins -- Prostaglandin
E1 promotes dilatation of the ductus arteriosus in infants with
ductal-dependent cardiac abnormalities.
Drug Name
Alprostadil (Prostaglandin
E1, Prostin) -- Causes relaxation
of smooth muscle, primarily within the ductus arteriosus. Used
in infants with ductal-dependent congenital heart disease due
to restricted systemic blood flow.
Pediatric Dose
0.01-0.1 mcg/kg/min IV infusion
Contraindications
Documented hypersensitivity;
respiratory distress syndrome or persistent fetal circulation
Interactions
Coadministration with heparin
may increase PTT or PT
Pregnancy
X - Contraindicated in pregnancy
Precautions
Closely monitor respiratory status,
cardiovascular status, and coagulation; apnea, fever, irritability,
and cutaneous flushing are common; inhibits platelet aggregation
Drug Category: Diuretic agents
-- These agents decrease preload by increasing free-water excretion.
Decreasing preload may improve systolic ventricular function.
Drug Name
Furosemide (Lasix)
-- Loop diuretic that blocks sodium reabsorption in the ascending
limb of loop of Henle.
Adult Dose
20-80 mg IV/IM/PO up to tid
Pediatric Dose
0.5-2 mg/kg IV/IM/PO up to tid
Contraindications
Documented hypersensitivity;
hepatic coma, anuria, and severe electrolyte depletion
Interactions
Antagonizes muscle-relaxing effect
of tubocurarine; auditory toxicity appears to be increased with
coadministration of aminoglycosides and furosemide; hearing
loss of varying degrees may occur; anticoagulant activity of
warfarin may be enhanced when taken concurrently with this medication
Pregnancy
C - Safety for use during pregnancy
has not been established.
Precautions
Profound diuresis and electrolyte
loss may result; metabolic alkalosis; use caution withother
medications known to decrease renal function; may cause hypercalciuria
and renal stones, especially in premature infants
Drug Name
Spironolactone (Aldactone) -- This
drug is a potassium-sparing loop diuretic.
Adult Dose
25-100 mg PO divided bid/qid
Pediatric Dose
2-3 mg/kg PO qd or divided bid
Contraindications
Documented hypersensitivity;
anuria, renal failure or hyperkalemia
Interactions
May decrease effect of anticoagulants;
potassium and potassium-sparing diuretics may increase toxicity
of spironolactone
Pregnancy
D - Unsafe in pregnancy
Precautions
Electrolyte imbalance, especially
hyperkalemia, may result; concomitant use with indomethacin
or ACE inhibitors may cause hyperkalemia
Drug Category: Cardiac glycosides
-- These medications improve ventricular
systolic function by increasing the calcium supply available
for myocyte contraction.
Drug Name
Digoxin (Lanoxin) -- This
form inhibits the sodium-potassium ATPase pump in cardiac myocytes.
Adult Dose
Total digitalizing dose (TDD):
1-1.5 mg PO given in divided doses over 1 d
Maintenance dose: 0.125-0.375 mg PO in 1-2 doses
Pediatric Dose
TDD: Premature infants: 0.02
mg/kg PO divided q8h for 3 doses
Full-term infants: 0.03 mg/kg PO divided q8h for 3 doses
1-24 months: 0.04-0.05 mg/kg PO divided q8h for 3 doses
>2 years: 0.03-0.04 mg/kg PO divided q8h for 3 doses
Maintenance dose:
Infants: 6-8 mcg/kg/d PO
2-5 years: 10-15 mcg/kg/d PO
5-10 years: 7 to 10 mcg/kg/d PO
>10 years: 3-5 mcg/kg/d PO
<10 years: bid dosing recommended
Contraindications
Documented hypersensitivity;
beriberi heart disease, idiopathic hypertrophic subaortic stenosis,
constrictive pericarditis, and carotid sinus syndrome
Interactions
Medications that may increase
digoxin levels include alprazolam, benzodiazepines, bepridil,
captopril, cyclosporine, propafenone, propantheline, quinidine,
diltiazem, aminoglycosides, oral amiodarone, anticholinergics,
diphenoxylate, erythromycin, felodipine, flecainide, hydroxychloroquine,
itraconazole, nifedipine, omeprazole, quinine, ibuprofen, indomethacin,
esmolol, tetracycline, tolbutamide, and verapamil
Medications that may decrease serum digoxin levels include aminoglutethimide,
antihistamines, cholestyramine, neomycin, penicillamine, aminoglycosides,
oral colestipol, hydantoins, hypoglycemic agents, antineoplastic
treatment combinations (including carmustine, bleomycin, methotrexate,
cytarabine, doxorubicin, cyclophosphamide, vincristine, procarbazine),
aluminum or magnesium antacids, rifampin, sucralfate, sulfasalazine,
barbiturates, kaolin/pectin, and aminosalicylic acid
Pregnancy
C - Safety for use during pregnancy
has not been established.
Precautions
Hypokalemia may reduce positive
inotropic effect of digitalis; IV calcium may produce arrhythmias
in digitalized patients; hypercalcemia predisposes patient to
digitalis toxicity, and hypocalcemia can make digoxin ineffective
until serum calcium levels are normal; magnesium replacement
therapy must be instituted in patients with hypomagnesemia to
prevent digitalis toxicity; patients with incomplete AV block
may progress to complete block when treated with digoxin; exercise
caution in hypothyroidism, hypoxia, and acute myocarditis
Drug Category: Inotropic agents --
These agents stimulate alpha-adrenergic
and beta-adrenergic and beta-dopaminergic receptors in the heart
and vascular bed.
Drug Name
Dopamine (Intropin) -- At
lower doses, stimulation of beta1-adrenergic and beta1-dopaminergic
receptors results in positive inotropism and renal vasodilatation;
at higher doses, stimulation of alpha-adrenergic receptors results
in peripheral and renal vasoconstriction.
Adult Dose
2-20 mcg/kg/min IV infusion
Pediatric Dose
Administer as in adults
Contraindications
Documented hypersensitivity;
pheochromocytoma or ventricular fibrillation
Interactions
Phenytoin, alpha- and beta-adrenergic
blockers, general anesthesia, and MAOIs increase and prolong
effects of dopamine
Pregnancy
C - Safety for use during pregnancy
has not been established.
Precautions
Use caution with intravascular
volume depletion; administration via a central venous catheter
is recommended; the umbilical artery should not be used; doses
higher than 20 mcg/kg/min generally are not helpful and other
agents should be considered; subcutaneous infiltration may cause
tissue sloughing; prompt treatment with subcutaneous phentolamine
(Regitine) is recommended
Drug Name
Dobutamine (Dobutrex) -- This
drug primarily stimulates the beta1-adrenergic receptor and
has less alpha-adrenergic stimulation, leading primarily to
increased myocardial contractility.
Adult Dose
2-20 mcg/kg/min IV infusion
Pediatric Dose
Administer as in adults
Contraindications
Documented hypersensitivity;
idiopathic hypertrophic subaortic stenosis and atrial fibrillation
or flutter
Interactions
Beta-adrenergic blockers antagonize
effects of dobutamine; general anesthetics may increase toxicity
Pregnancy
B - Usually safe but benefits
must outweigh the risks.
Precautions
Use caution with intravascular
volume depletion; administration via a central venous catheter
is recommended; the umbilical artery should not be used; doses
higher than 20 mcg/kg/min generally are not helpful, and other
agents should be considered; subcutaneous infiltration may cause
tissue ischemia
Drug Category: Afterload-reducing
agents -- Afterload reduction improves myocardial performance
and theoretically reduces atrioventricular and semilunar valve
insufficiency.
Drug Name
Captopril (Capoten) -- ACE
inhibitor, which decreases the production of angiotensin II,
a potent vasoconstrictor, resulting in peripheral vasodilatation
and afterload reduction, improving myocardial performance and
theoretically reducing AV and semilunar valve insufficiency.
Administer a test dose of 0.1 mg PO to assess initial response
Adult Dose
6.25-12.5 mg PO tid; not to exceed
150 mg tid
Pediatric Dose
0.1-1 mg/kg PO tid
Contraindications
Documented hypersensitivity;
renal impairment
Interactions
NSAIDs may reduce hypotensive
effects of captopril; ACE inhibitors may increase digoxin, lithium,
and allopurinol levels; rifampin decreases captopril levels;
probenecid may increase captopril levels; the hypotensive effects
of ACE inhibitors may be enhanced when given concurrently with
diuretics
Pregnancy
C - Safety for use during pregnancy
has not been established.
Precautions
Pregnancy category D in second
and third trimesters; caution in renal impairment, valvular
stenosis, or severe congestive heart failure; profound hypotensive
response is observed rarely after the initial dose in smaller
children; an initial test dose should be given and blood pressure
should be monitored carefully; dose should be titrated based
on clinical response and tolerance; use caution with decreased
renal function; ACE inhibitors have a potassium-sparing effect
when administered with furosemide; simultaneous administration
of spironolactone should be done with caution
Drug Category: Antiplatelet agents
-- These agents are used in the
treatment or prevention of thrombo-occlusive disease mediated
by the action of platelets. They inhibit platelet function by
blocking cyclooxygenase and subsequent aggregation.
Drug Name
Aspirin (Anacin, Ascriptin, Bayer Aspirin)
-- Inhibits the enzyme cyclooxygenase
that reduces production of thromboxane A2, which is a potent
vasoconstrictor and platelet-aggregating agent. Antiplatelet
effects of aspirin last the entire life of the platelet (6-10
d) and are not reversible.
Adult Dose
325 mg PO qd
Pediatric Dose
5-10 mg/kg PO qd
Contraindications
Documented hypersensitivity;
liver damage, hypoprothrombinemia, vitamin K deficiency, bleeding
disorders, asthma; because of association of aspirin with Reye
syndrome, do not use in children (<16 y) with flu
Interactions
Effects may decrease with antacids
and urinary alkalinizers; corticosteroids decrease salicylate
serum levels; additive hypoprothrombinemic effects and increased
bleeding time may occur with coadministration of anticoagulants;
may antagonize uricosuric effects of probenecid and increase
toxicity of phenytoin and valproic acid; doses >2 g/d may
potentiate glucose-lowering effect of sulfonylurea drugs
Pregnancy
D - Unsafe in pregnancy
Precautions
May cause transient decrease
in renal function and aggravate chronic kidney disease; avoid
use in patients with severe anemia, with a history of blood
coagulation defects, or who are taking anticoagulants
Followup:
Further Inpatient Care:
* Initial preoperative management and
postoperative care take place in the neonatal, pediatric, or
cardiac ICUs.
* When postoperative patients are clinically
stable, transfer them to the general cardiac unit for adjusting
oral medications, addressing feeding issues, and completing
discharge teaching.
* Involve a pediatric cardiologist during
any noncardiac hospital admission of a patient who is status
post (S/P) Norwood procedure. This is because of the complex
cardiovascular physiology in infants after this surgery.
Further Outpatient Care:
* Schedule outpatient follow-up care
2 weeks after discharge in the typical postoperative patient.
* Schedule those who are S/P cardiac transplantation
earlier for necessary laboratory studies.
* Earlier follow-up care is also necessary
if a pericardial effusion is discovered on the discharge echocardiogram.
* Individualize further outpatient follow-up
care based on the needs of each patient.
In/Out Patient Meds:
* Inpatient medications
o Prostaglandin E1
o Dopamine/dobutamine
o Furosemide (Lasix/Aldactone)
o Captopril
o Digoxin
* Outpatient medications
o Furosemide (Lasix/Aldactone)
o Captopril
o Digoxin
Transfer:
* Transfer the infant to a hospital
with appropriate ICUs. Pediatric cardiology and cardiovascular
surgery services must be immediately available.
* Carefully monitor the infant for apnea
during transfer while on prostaglandin E1 therapy. If prostaglandin
E1 has been started, consider elective endotracheal intubation
before transfer.
Complications:
* Preoperative complications include
acidosis, CHF, renal failure, liver failure, necrotizing enterocolitis,
sepsis, and death.
* Postoperative complications include acidosis,
CHF, renal failure, liver failure, necrotizing enterocolitis,
sepsis, pericardial or pleural effusion, phrenic or recurrent
laryngeal nerve damage, stroke, coarctation of the aorta, and
death. Early graft rejection and opportunist infection may occur
after cardiac transplantation.
* Major complications following the Norwood
procedure include aortic arch obstruction at the site of surgical
anastomosis and progressive cyanosis caused by limited blood
flow through the shunt. An inadequate atrial communication contributes
to progressive cyanosis.
* Major complications following the hemi-Fontan
procedure include transient superior vena cava syndrome and
persistent pleural or pericardial effusion. The development
of systemic venous to pulmonary venous collateral vessels is
possible.
* Major complications following the Fontan
procedure include persistent pleural or pericardial effusion.
Neurodevelopmental abnormalities are reported and may be inherent
in some patients with hypoplastic left heart syndrome.
Prognosis:
* Overall survival to the time of hospital
discharge after the Norwood procedure is nearly 75%. Success
rates are higher in uncomplicated cases and lower in cases in
which important preoperative risk factors are present, such
as age greater than 1 month, significant preoperative tricuspid
insufficiency, pulmonary venous hypertension, associated major
chromosomal or noncardiac abnormalities, and prematurity.
* Survival after the hemi-Fontan and Fontan
operations is nearly 90-95%.
* The actuarial survival rate after staged
reconstruction is 70% at 5 years.
* Institutional success rates vary.
* Neurodevelopmental prognosis is not known;
however, abnormalities are reported.
* Approximately 20% of infants listed for
cardiac transplantation die while waiting for a donor heart.
After successful transplantation, the survival rate at 5 years
is approximately 80%.
* When the preoperative mortality is considered,
the overall survival rate after cardiac transplantation is approximately
70%, or similar to the results for staged reconstruction.
Patient Education:
* Medication
o Educate parents regarding the doses
and side effects of their child's cardiac medications.
o Discuss interactions with other medications with the family
and the infant's general pediatrician.
* Feeding
o Many infants require nasogastric
tube feeding after discharge from the hospital. Parents must
become comfortable with placement of the nasogastric feeding
tube.
o Frequently, increased-calorie formula
is required for adequate growth. Provide the formula recipe
or a source for purchasing it to the caregiver.
* Follow-up care
o Stress the importance of
follow-up care. If necessary, provide cab or bus vouchers to
ensure compliance.
o If noncompliance becomes a critical issue, physicians are
required to report to the appropriate family services agency.
Medical/Legal Pitfalls:
* Misdiagnosis
* Failure to recognize the infant with hypoplastic
left heart syndrome and the obstruction to pulmonary venous
return
Special Concerns:
* The newborn with hypoplastic left
heart syndrome dies rapidly if untreated. Surgical techniques,
both reconstruction and heart transplantation, offer an opportunity
to preserve the newborn's life. Survival rates given above represent
the best results and reflect only survival, not quality of life.
Mortality rates in many centers exceed those mentioned. Incidence
of neurodevelopmental abnormalities in hypoplastic left heart
syndrome appears to exceed that of other single-ventricle conditions.
* Hypoplastic left heart syndrome affects
family structure. For example, reproductive studies indicate
that the incidence of subsequent pregnancy is significantly
lower in mothers of a living patient with hypoplastic left heart
syndrome than in mothers after death of an infant with hypoplastic
left heart syndrome. For these reasons, most pediatric cardiologists
continue to offer no treatment as an acceptable option to parents
of a newborn with hypoplastic left heart syndrome. It is incumbent
on physicians caring for a newborn with hypoplastic left heart
syndrome to clearly communicate all of this information to the
parents. An ethically appropriate consent for surgery requires
this. Allowing an affected infant to die without surgical intervention
is a difficult decision, but it is still chosen by some families.