Pulmonary stenosis occurs in 10-12% of cases of congenital heart
disease in adults. The obstruction is vascular in 90% of patients,
but may occur above or below the valve itself. There may be
associated other congenital heart defects. Although the three
valve cusps (see figure 25) in stenosis
are usually thin and pliant, their commissures are fused (see
figure 25), leading to a dome-shaped
valve with a small central opening during ventricular contraction
(systole). The other 10% of cases have thickened, immobile and
myxomatous. The normal valve area is 2.0 cm2 per square meter
of body surface area, with no pressure gradient across the valve
during systole. When the valve becomes stenotic, the right ventricle
systolic pressure increases, creating a gradient across the
valve. Pulmonary stenosis is mild, if the valve area is larger
than 1.0 cm2 per square meter and the trans-valvular gradient
is 50-80 mmHg, or the peak RV systolic pressure is less than
75 mmHg. The stenosis is moderate if valve area is 0.5-1.0 cm2
per square meter, trans-valvular gradient is 50-80 mmHg, or
right ventricle systolic pressure is 75-100 mmHg. It is severe
when the valve area is less than 0.5 cm2, and the gradient is
more than 80 mmHg.
Diagnosis
includes the following:
1)
Physical examination. A thrill may be felt along the left sternal
border. A murmur may be heard along the left sternal border.
The murmur comes from a narrowing of a segment
of the pulmonary artery above the pulmonary valve or the narrowing
can be in one of the pulmonary artery branches (right or left).
The murmur is a harsh noise peaking in the middle of the cycle
of the heart contracting to push blood through the pulmonary
artery. The blood going through a narrowed segment of the pulmonary
artery creates this noise, best heard just to the left of middle
line of the chest, up close to and under the left collar bone
(clavicle) and can also be heard under the left arm and in the
back!
2)
EKG show right ventricular wall thickening (RVH).
3)
Echocardiogram show right ventricular wall thickening (RVH)
and paradoxically septal (IVS) motion during systole, and the
site of obstruction. Doppler studies can assess the degree of
stenosis.
The clinical course of pulmonary stenosis
is favorable in most patients
with mild to moderate obstruction. In a national study, 86%
of patients had no increase in their pressure gradients over
a 4- to 8-year interval. Those with a significant increase were
less than 4 years of age and had at least moderte stenosis initially.
Progression during the period of growth
seems to be the likely explanation for most of the increases,
but a few patients developed subvalvular muscular hypertrophy,
which increased the obstruction.
Even mild obstruction may progress significantly
in some infants during
the first year of life. The prognosis of those with severe obstruction
without intervention is poor, especially in infants with critical
obstruction. With severe obstruction, right ventricular damage
and dysfunction can ensure over the years, and heart failure
or arrhythmias can cause premature death in adults.Tricuspid
regurgitation also may result. Obstructon of the subvalvular
type frequently increases with time. Brain abscess, infective
endocarditis may occur.
In the above cited national study reevaluated
15 to 25 years later, the probability of 25-year survival was
95.6% compared with an expected age- and sex-matched control
group survival of 96.6%.97% were asymptomatic. Studies suggested
no pulmonary stenosis in 2 %, mild stenosis in 93%, moderate
in 3%, and severe stenosis in only 1%
Treatment depends on the degree of stenosis
.Frequent reexaminations
are indicated to detect any evidence of progression, with more
frequent
evaluation for those under one year of age.
Balloon valvuloplasty has replaced surgery
therapy as a first approach (see attached figures).
A thickened, immobile, dysplastc pulmonary
valve is best treated by
complete excision. Sub valvular stenosis is relieved through
a right
vehtriculotomy, a main pulmonary arteriotomy or a right atriotomy.
The blueness of the eyelids may represent
cyanosis (desaturation of the
blood due to venous blood being mixed through a shunt like the
atrial septal defect with oxygenated blood in the left atrium)
and should be brought to the cardiologist's attention.
As an illustration of the use of stents in pulmonary artery
branch stenosis, which had developed following surgical repair
that was refractory to balloon dilatation, the following article
by V.Mercieca,V.Grech and JV DeGiovanni in Images in Paediatric
Cardiology(2004;20:1-10) is presented below.
Introduction
Congenital pulmonary artery (PA) branch stenosis can occur in
isolation, as part of a syndrome or in conjunction with other
cardiac defects; quite often, PA branch stenosis occurs after
surgical repair of congenital heart disease. Significant narrowing
of the pulmonary artery origins can lead an overall reduction
in pulmonary blood flow or to disproportionate distribution
to the two lungs. In addition, an increase in right ventricular
systolic pressure will result in right ventricular hypertrophy
and possible failure. Moreover, coexistence of pulmonary regurgitation
is made worse by PA branch stenosis. A right ventricular peak
systolic pressure equal to or greater than 50% of the aortic
systolic pressure or quantitative pulmonary perfusion scans
showing ipsilateral lung perfusion <35% than predicted in
unilateral stenoses are indications for intervention.
Percutaneous transluminal balloon angioplasty for PA branch
stenosis is frequently of limited effectiveness in decreasing
the pressure gradient, may have significant complications such
as vessel rupture, dissection, aneurysm formation or even death
and is often followed by recurrence. The latter has become less
problematic with the advent of balloon expandable stents. The
radial force of the stent holds the vessel open after deployment,
counteracting the natural elastic recoil of the arterial wall.
This elastic recoil of the vessel also helps to anchor the stent
in place until epithelisation occurs. The collapsed stent is
introduced over a deflated balloon catheter into the femoral
vein through a long and wide Mullins sheath. Once the stent
has been correctly positioned the balloon is inflated to deliver
the stent over the stenosis in the vessel. The balloon is then
deflated and withdrawn. Balloon expandable stents have added
advantage that they can be further dilated when necessary as
may be the case with growing children or in case of subsequent
restenosis.
We present a patient with tetralogy of Fallot who developed
bilateral branch pulmonary artery stenosis following surgical
repair that was refractory to balloon dilatation. We describe
simultaneous stenting of both PAs with a pleasing result.
Patient
Our patient, a 16 year old female had a repair of tetralogy
of Fallot at the age of 14 months following a severe cyanotic
spell. At operation the ventricular septal defect was closed
with a Gortex patch and infundibular resection was carried out
together with a trans-annular patch. She was left with a small
residual ventricular septal defect which closed spontaneously,
pulmonary regurgitation and mild residual infundibular stenosis.
Postoperatively echocardiography demonstrated a dilated main
pulmonary artery
and narrowing of the origin of the RPA with marked turbulence
on colour flow mapping and peak Peak Doppler gradients were
up to 60 mm Hg.
Fifteen years after surgery, balloon angioplasty was carried
out for bilateral branch PA stenosis. The right had two levels
of stenosis: main pulmonary artery (MPA) to distal right pulmonary
artery (RPA) gradient 22 = mm Hg) and the left was tightly stenosed
at its origin: MPA to left pulmonary artery (LPA) gradient =
30 mm Hg. The procedure did not produce any significant amelioration
in gradients or angiographic appearance.
A further intervention was carried out at a later date with
the intention to place stents to the origins of both PAs. Access
to the LPA was from the right femoral vein and the RPA from
the left femoral vein and a superstiff wire was used on each
side.
Figure 1: (click here for video)
RV angiogram — note RPA and LPA stenoses (arrows)
Figure 2: (click here for video)
MPA angiogram further delineating LPA stenosis
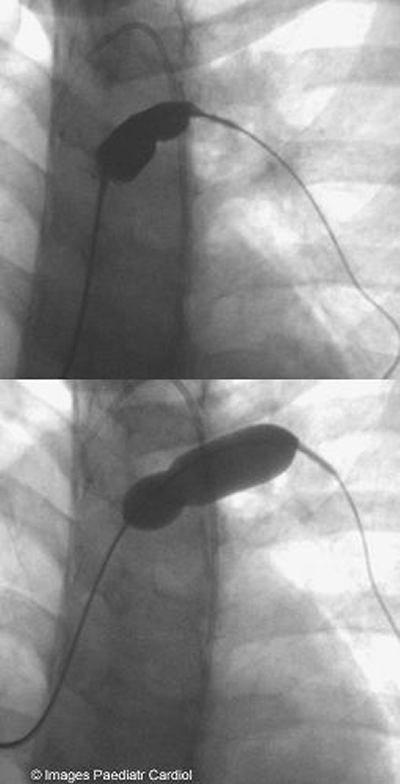
Figure 3: Predilatation of LPA
As the LPA stenosis was complex and severe, it was decided
to predilate with a high pressure balloon prior to stenting(figure
3).
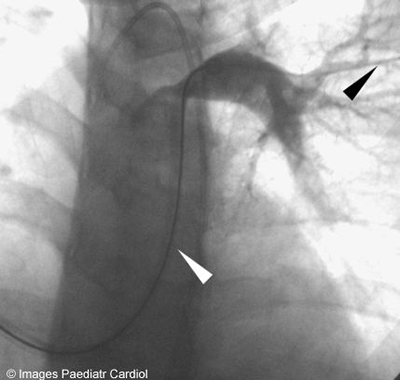
Two separate Mullins sheaths sizes(11F on the left and 12F
on the right side) were introduced in the PAs distal to the
stenosis(figure 4).
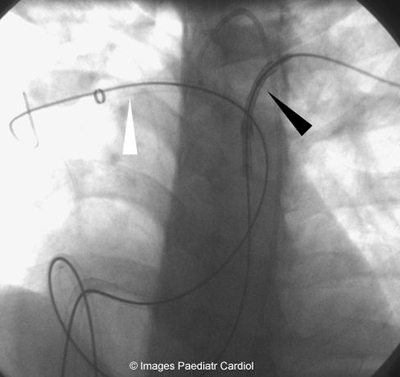
Figure 5: Mullins sheaths in both branch PAs — stent mounted
over balloon being delivered to the
LPA
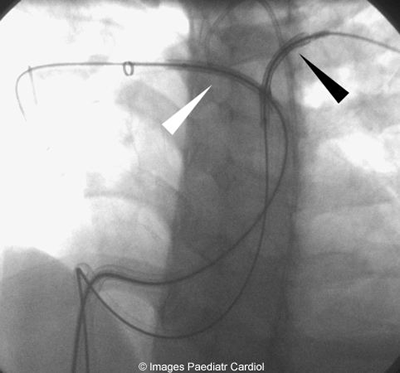
Figure 6: Both stents being placed prior to deployment
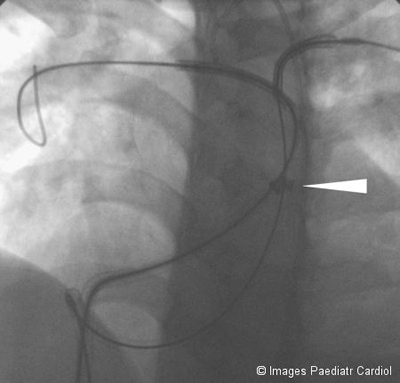
Figure 7: Both Mullins sheaths withdrawn (tip of sheaths indicated
by arrows) exposing both stents
Two 59 mm Genesis Cordis stents were used. The RPA stent was
deployed on an 18mm Crystal balloon while a 16 mm MaxiLD balloon
was used for the LPA stent. Precise positioning of the stents
was evaluated by angiography through the Mullins sheath. Simultaneous
inflation of the balloons by 2 operators working synchronously
led to the deployment of the 2 stents at the same time; this
is a crucial part of the technique in order to avoid displacement
of one of the stents or occlusion of a PA branch. The anaesthetist
provided a short period of apnoea during balloon inflation in
order to minimise balloon movement or displacement.
Figure 8: (click here for video)
Simultaneous inflation of both stents
The proximal and distal ends of the stents were flared open
by inflating the balloon distally and proximally.
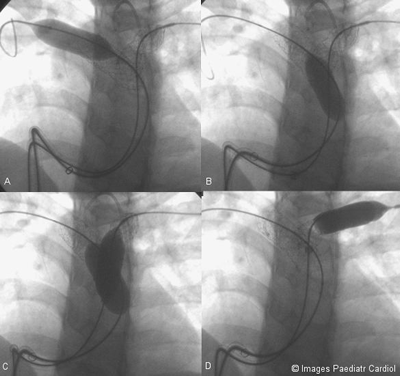
Figure 9: Proximal and distal ends of both stents being flared
open. A. Distal end of RPA stent B.
Proximal end of RPA stent C. Proximal ends of both stents D.
Distal end of LPA stent
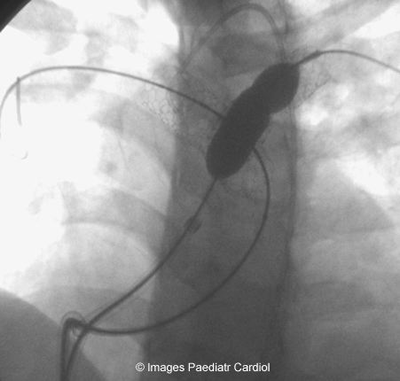
Figure 10: LPA stent being reinflated
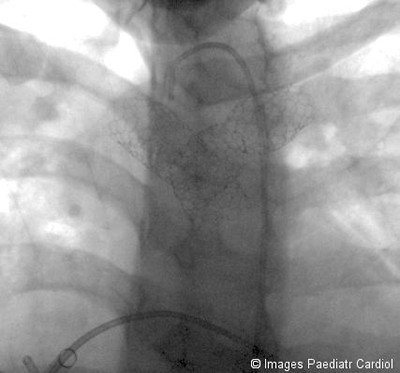
Figure 11: Final result
Figure 12: (click here for
video) Final angiographic result
The gradients across the PAs on this occasion fell from 42
to 10 mm Hg on the right and from 50 to 8 mm Hg on the left.
RV pressure fell from 70/- to 32/- following the procedure.
There were no complications.

Figure 13: Echocardiographic parasternal short axis view showing
A. RPA stent B. LPA stent.
Note stent mesh structure clearly visualised on 2D echocardiography
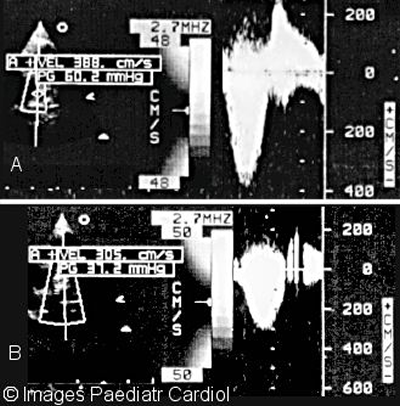
Figure 14: Continuous wave Doppler of right ventricular outflow
tract and LPA showing A. Peak gradient before (60 mmHg) and
B. Peak gradient after (37 mmHg)
Conclusion
Bilateral PA origin stenosis may require stenting and this should
be done simultaneously. Pre-dilatation may be indicated in some
cases and stability of the stents prior to deployment is essential
using superstiff wires, apnoea and, if the degree of pulmonary
regurgitation is severe, consider a large dose of adenosine,
esmolol infusion or fast pacing.
References
1. Chandar JS, Wolfe SB, Rao PS. Role of stents in the management
of congenital heart defects. J Invasive Cardiol 1996;8:314—325
2. Shaffer KM, Mullins CE, Grifka RG. Intravascular Stents in
congenital heart disease: short and
long-term results from a large single-center experience. J Am
Coll Cardiol 1998;31:661-667
3. Ing FF Grifka RG Nihill MR Mullins CE. Repeat dilation of
intravascular stents in congenital
heart defects. Circulation 1995;92:893-897
4. Rao PS. Stents in the management of congenital heart disease
in pediatric and adult patients.Indian Heart J. 2001;53:714-730