This
condition accounts for a third of the adult cases of congenital
heart disease, occurring two to three times more frequent in
women.
It may occur in various positions in the atrial septum (see
figure 20):
1) lower part, ostium primum, 15% of cases;
2) ostium secundum, in area of fossa ovalis (prior site of foramen
avalis in the fetus, allowing both left and right atrium to
communicate), 75%;
3) upper atrial septum, sinus (site of sinus or pocket where
inferior vena cava (IVC) and superior vena cava (SVC) empty
into right atrium) venosus, 10%.
Most
cases are due to spontaneous genetic mutations, but others are
inherited.
The results of these defects come from the shunting of blood
from one atrium to the other.
The
direction and size of the shunting are determined by the size
of the defect and compliance of the ventricles.
A small defect less than 0.5 cm in diameter is associated with
a small shunt and no significant sequelae.
But a larger defect, more than 2 cm in diameter may be associated
with a large shunt with important blood flow changes.
In most cases with atrial defects, the right ventricle is more
flexible than the left; thus, the left atrial oxygenated blood
is shunted to the right atrium causing increased blood flow
and enlargement of the atria, right ventricle, and pulmonary
arteries (see figure
112a ).
But if the right ventricle fails, the shunt may reverse and
go right to left.
Diagnosis
includes the following:
1) Auscultation of the heart sounds and searching for heart
murmurs
2) EKG
3) Echocardiography including transesophageal, and doppler (
Figure
112b )
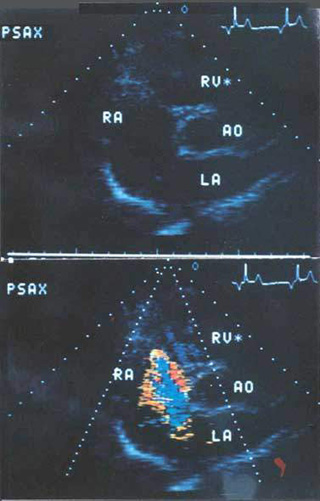
4) Heart catherterization to determine size and direction of
shunt and whether there is high blood pressure in the pulmonary
arteries.
Patients with moderate or even large atrial septal defects often
have no symptoms until 30 to 40 years of age.
Ultimately, with the increased blood flow through the right
heart chambers, right ventricular enlargement and failure occurs.
Symptoms include fatigue and shortness of breath on exertion.
Also, supraventricular arrhythmia like atrial tachycardia (see
figure
1) and fibrillation (see figure
14), heart failure, and embolism through the defect may
occur and can result in death.
An example of a complication of a paradoxical
embolism in a case of a patent foramen ovale is illustrated
below(New England Journal of Medicine 2007;357(22):2285):
Supplement
to: Dörr M and Hummel A. Paradoxical Embolism — Thrombus
in a Patent Foramen Ovale. N Engl J Med 2007;357(22):2285.
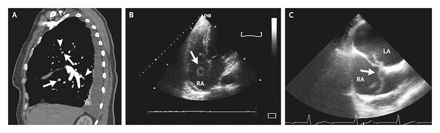
An 85-year-old woman was admitted with progressive
dyspnea and chest pain that had started suddenly 2 days earlier.
Physical examination revealed formerly undiagnosed atrial fibrillation
with a normal heart rate and normal blood pressure. Examination
of the lungs did not show any pathological findings. Pulmonary
embolism was diagnosed on spiral computed tomography (Panel
A, sagittal reconstruction), which showed intraluminal filling
defects (arrows) and total occlusions of the upper and lower
segmental arteries by clots (arrowheads). Duplex ultrasonography
revealed an underlying deep-vein thrombosis of the right superficial
femoral vein. Transthoracic echocardiography showed typical
signs of moderate right heart strain. Anticoagulation therapy
with low-molecular-weight heparin was started. One week later,
repeated transthoracic echocardiography showed a fluttering
thrombus within the right atrium (Panel B, arrow). Two-dimensional
transesophageal echocardiography revealed a vermicular thrombus
trapped in the patent foramen ovale with floating parts in the
right and left atrium (Panel C, arrow, and video clip, available
with the full text of this article at www.nejm.org). Emergency
cardiac surgery was performed in which a thrombus of approximately
8 cm in length was extracted and the foramen ovale was closed.
Surprisingly, the left atrial fraction of the thrombus had already
disappeared without clinical signs of arterial embolism. The
patient was discharged in good clinical condition 2 weeks later
while receiving anticoagulant therapy for persistent atrial
fibrillation. Ten months later, she was still doing well without
relapse.
see video
This video is copyright protected, not downloadable and can be found in the New England Journal of Medicine, issue November 29, 2007, page , volume , in the article called Paradoxical Embolism. It is shown here to point out the embolus's fragile, serpigenous, loosely structured, thin , floating, mobile state(altered by the periodic contractions of the heart) caught in the hole (Foramen Ovale) connecting the two atria.
Treatment
If
the blood flow through the pulmonary artery is 1.5 times larger
than the flow out of the left ventricle, surgical closure of
the defect is recommended.
But surgery is not recommended for patients with irreversible
pulmonary arterial disease and high pulmonary blood pressure.
Defects of the secundum type of atrial septal
defect usually go undetected in the first year or two of life
because of the lack of symptoms and unimpressive auscultatory
findings. A soft systolic murmur is the usual reason for referral.
Symptoms become more common in persons in their late teens and
twenties, and by age 40 the majority of these individuals are
symptomatic, some severely so.
Spontaneous closure of these defects is rare beyond the first
two years of life. Transcatheter closure of centrally located
secundum in selected older infants, children and adults using
a double umbrella or a buttoned device appears to be an acceptable
alternative to surgical closure(i.e.the Amplatzer septal occluder).
THE AMPLATZER SEPTAL OCCLUDER
http://www.amplatzer.com/images/asdsmall.jpg
Device Description
The Amplatzer septal occluder is a self-expandable, double
disc device made from a Nitinol wire mesh. The two discs are
linked together by a short connecting waist corresponding to
the size of the ASD. In order to increase its closing ability,
the discs and the waist are filled with polyester fabric. The
polyester fabric is securely sewn to each disc by a polyester
thread.

asdsmall Fig.1
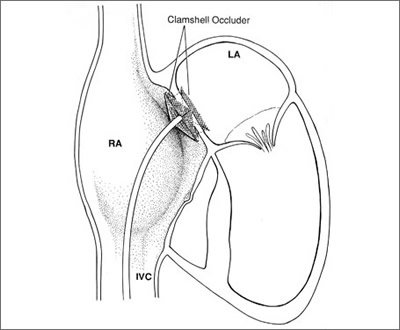
clamshell device atrial septal defect Fig.1a
The Amplatzer delivery system was designed specifically to
facilitate attachment, loading, delivery and deployment of the
Amplatzer septal occluder and is comprised of a delivery sheath,
dilator, loading device, plastic vise and delivery cable.
TRANSCATHETER CLOSURE OF ATRIAL SEPTAL DEFECT
Isolated atrial septal defect accounts for approximately 6%
of CHD. The pathophysiology is that of a low-pressure left-to-right
shunt with right heart dilation and pulmonary overcirculation.
Symptoms are rare before the third decade and include CHF, pulmonary
hypertension and atrial arrhythmia. Rarely a very large defect
may cause CHF with growth failure in a young child. It is difficult
to predict which patients will go on to develop symptoms. Not
all symptoms are reversible if the defect is repaired in adulthood.
For these reasons, moderate to large defects found in young
children are usually closed before the child starts school or
as soon as they are found in older children.
Surgical closure performed since 1948 enjoys excellent results
and very low morbidity and mortality. A sternal or more uncommonly
thoracic incision is required as is cardio-pulmonary bypass
and a three-day to a one-week hospitalization.
King et al. reported the first transcatheter closure of an
ASD in 1976 using a device that required an extremely large
sheath (or catheter) for implantation. Since then, several different
designs have undergone and continue to undergo clinical testing
with generally good results. As of this writing, there are several
active clinical trials in the United States and abroad using
devices such as the Amplatzer, the CardioSeal, and the Angel
Wings. Although varying in specific details of construction,
most devices have in common two discs connected together in
the center and designed so that they can collapse to fit inside
a catheter and expand again when positioned in the heart.
In the typical "clamshell" design, two large discs
positioned on either side of the defect are responsible for
closure of the defect. Potential disadvantages of this design
include the need for large discs relative to the size of the
defect and—because of the lack of a centering mechanism—the
technical difficulty in getting the device to sit properly within
the defect. The Amplatzer ASD occluder is an example of the
"self-centering design" where the connection between
the two discs is larger in diameter and serves to center the
device within the defect and also aids in occlusion (asdwax-Fig1b.jpg).
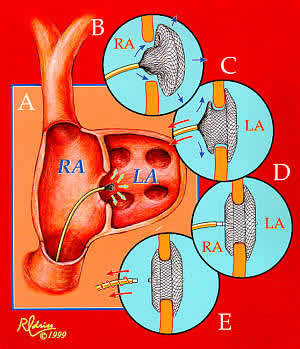
asdwax-Fig1b.jpg
Implantation of the Amplatzer ASD occluder. From A to E: The
delivery catheter is positioned across the atrial defect; the
left atrial disc with the self-centering connecting stalk is
delivered; the device is withdrawn so that the connecting stalk
is within the ASD and the left disc is firm against the atrial
septum; the right atrial disc is delivered, and the delivery
cable is disconnected from the device. Until the delivery cable
is disconnected, the device can be withdrawn back into the catheter
and removed from the body.
The Amplatzer has been used in over 2,700 patients world wide
since 1995 .The Amplatzer offers several advantages over competing
designs. Because of its Nitinol (memory wire) construction and
unique design it can be withdrawn back into the catheter and
removed from the body if proper positioning in the heart is
not possible. The device is available in a range of sizes from
4 mm to 32 mm; the appropriate size allows the connecting stalk
to fill the ASD, center the device, ensure complete closure
and allow the atrial discs to be relatively smaller than those
of competing designs.
asdwax-Fig1b.jpg above demonstrates the technique for inserting
the Amplatzer. The procedure is currently performed under general
anesthesia to allow for transesophageal echo during the procedure.
The patient is observed overnight and discharged the next morning.
Patients take a baby aspirin daily for six months until endothelialization
of the device is complete. Follow-up consists of an echocardiogram
and a chest x-ray at six months and one year. Possible complications
include infection at the catheter site, arrhythmia, stroke,
cardiac perforation, device embolization and incomplete closure.
Most, but not all, secundum atrial septal defects are amenable
to closure by the Amplatzer device. Currently criteria include
a defect size of less than or equal to 32 mm and a 4 mm rim
of atrial septal tissue surrounding the defect. These anatomical
details can usually be ascertained by a careful transthoracic
echocardiogram. Occasionally, in larger patients a transesphogeal
echo is needed to properly visualize the defect. If this is
required, we offer patients the option of having the TEE under
general anesthesia with catheter closure of the defect to follow
if appropriate.
At the last reporting of U.S. results, 186 patients had atrial
defects closed with the Amplatzer device with no major complications.
There were 8 (4.3%) minor complications including 4 device embolizations
(2 removed percutaneously and 2 removed surgically) and 4 instances
of arrhythmia (3 transient, 1 persistent complete heart block).
The closure rate for the 121 patients with 6 months follow-up
was 99%.
Therapeutic cardiac catheterization in children :DAVID F. WAX,
MD;Fall 1999
http://www.childsdoc.org/Fall99/catheterization.asp
Sandwich cookie figure1c analogy of Amplatzer device:The two
cookies form the two slices of the sandwich and the atrial septal
defect the creme .
The risk of surgery are minimal (>than 0.5%) with all these
children home by the fourth postoperative day. Morbidity in
adults and the low risk of surgical closure in young children
mandate surgery in the preschool or preadolescent years.
Although life-threatening complications after closure of ASDs
are rare, transient postoperative atrial arrhythmias and postpericardiotomy
syndrome with pericardial effusions occasionally seen. The long
term prognosis for a normal life expectancy and functional capability
is excellent for patients who have closure of an uncomplicated
ASD during the first two decades of life.
MEDICAL MANAGEMENT
The few infants who present with symptoms
of congestive failure are treated with digoxin and, if necessary,
diuretics and are studied by cardiac catheterization. If the
defect is uncomplicated and the symptoms persist despite a trial
of therapy, surgical closure is advised without further delay.
For asymptomatic infants and children, closure is recommended
just before entry into school. Restrictions of activity or exercise
are unnecessary. If the physical, laboratory, and echocardiographic
findings are completely characteristic, preoperative catheterization
is not necessary. Closure is recommended if the defect is large
and if there is right ventricular volume overload or (ecbocardiography.
In those with puImonary hypertension closure is recommended
for patients with Qy/Q, ratios >1.5:1 by catheterization
provided that the systemic arterial saturation is >92 percent
and total Rf < 15 Wood units. Closure would seem prudent
before pregnancy or the use of contraceptives in view of the
tendency to develop rapidly progressive PVOD in this setting.
Transcatheter closure of centrally located
secundum in selected older infants, children, and adults using
a double-umbrella ("clamshell") or a buttoned device
appears to be an acceptable alternative to surgical closure(see
above).. Infective endocarditis is rare, and antibiotic coverage
at times of possible bacteremia is recommended only if associated
mitral value disease is suspected.
Below is a summary of a recent article appearing in the JACC,17
January 03 issue describing the safe use of intracardiac echocardiography
to guide the placement of the Amplatzer septal occluder over
atrial septal defects successfully without general anesthesia
,which is required with transesophageal echocardioigraphy ,allowing
the patient to be discharged the same day as the procedure.
INTRACARDIAC ECHOCARDIOGRAPHIC-GUIDED DEVICE CLOSURE OF ATRIAL
SEPTAL DEFECTS
Michael J. Mullen, MD, MRCP,* Bryan F. Dias, MD,* Fiona Walker,
MD, MRCP,*
Samuel C. Siu, MD, SM,* Lee N. Benson, MD, FACC,t Peter R. McLaughlin,
MD, FACC*
Toronto, Canada
OBJECTIVES This study was designed to determine the feasibility
and accuracy of intracardiac echocardiography, (ICE) in guiding
percutaneous closure of atrial septal defects (ASD).
BACKGROUND Intracardiac echocardiography is a novel imaging
technique that might be used to guide interventional procedures.
The sensitivity and specificity of ICE, compared to standard
imaging techniques, in detecting potentially adverse procedural
events and guiding remedial action will be an important consideration
in its use.
METHODS In a prospective study, 24 patients underwent device
closure of ASD using ICE as the primary echocardiographic imaging
modality. Feasibility was expressed as proportion of cases in
which complete diagnostic ICE imaging was achieved. Accuracy
was expressed as the percent agreement between ICE and simultaneously
performed transesophageal echocardiography (TEE).
RESULTS Hligh-quality ICE images were acquired in all patients,
though images were limited in two patients with aneurvsmal septa(See
figures below). Intracardiac echocardiography successfully guided
closure of 24 out of 25 ASDs (96%) in 23 patients. There was
close agreement between ICE and TEE in their assessment of device
position and the adequacy of septal capture before device release
(98%) and in identifying the presence of significant residual
shunts. Intracardiac echocardiography detected all potentially
adverse events, including four malpositions, and guided appropriate
remedial action.
CONCLUSIONS Intracardiac echocardiography guided device closure
of secundum ASDs is feasible in the majority of patients and
provides diagnostic data comparable to TEE. These data indicate
that ICE may be used to guide routine closure of ASDs in adults
without the need for TEE and general anesthesia. JAm Coll Cardiol
2003;41:285-92) © 2003 by the American College of Cardiology
Foundation
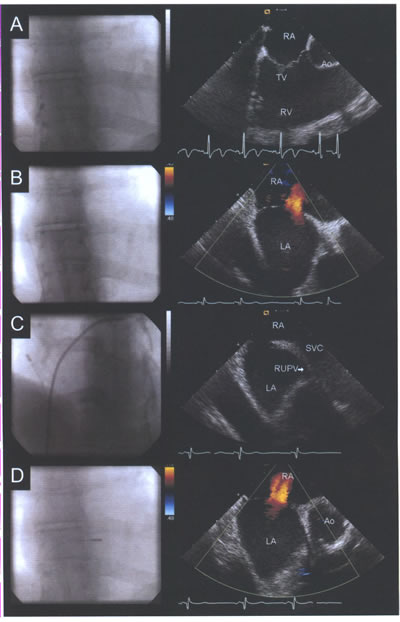
asdICE-Fig16.jpg: Fluoroscopic position of the intracardiac
transducer with corresponding echocardiographic images. (A)
Long-axis view of the tricuspid valve (TV), right ventricle
(RV), and right ventricular outflow tract viewed from the right
atrium (RA). (B) Rotation of the catheter clockwise reveals
a long-axis view of atrial septum and the left atrium (LA);
color Doppler demonstrates an atrial septal defect. (C) Advancing
the transducer cranially with slight posterior flexion reveals
the sinus venosus septum, superior vena cava (SVC), and origin
of the right upper pulmonary vein (RUPV). (D) Further posterior
flexion and rotation of the transducer towards the TV provides
a short axis image of the aorta (Ao), atrial septum, and atrial
septal defect.
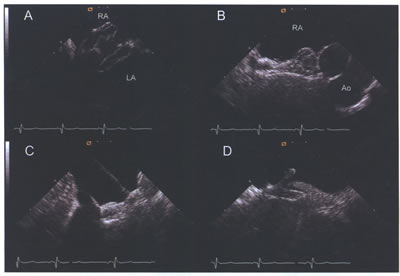
asdICE-Fig17.jpg (A) Amplatzer septal occluder with both left
atrial (LA) and right atrial (RA) discs visible during deployment.
(B) Short-axis view of Amplatzcr device following deployment.
(C and D) Long-axis views illustrating capture of the membranous
inferior and muscular superior septum.
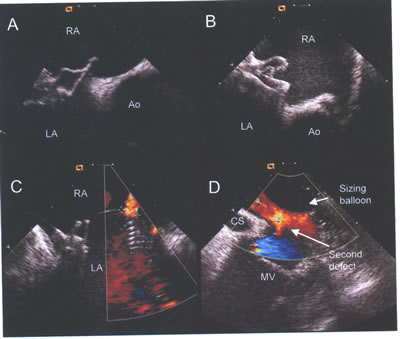
asdICE-Fig18.jpg. Adverse procedural events detected by intracardiac
echocardiography. (A and B) Malpositioned Cardioscal and Amplatzer
devices with both discs on right atrial (RA) side of aorta (Ao).
(C) Color flow demonstrates a residual leak in this patient,
with two devices already deployed. Intracardiac echocardiography
demonstrated this to be a small additional fenestration. (D)
This second large inferior defect was not identified bytransesophageal
echocardiograhy.Note the sizing ballon inflated within a more
superior defect and the coronary sinus(CS) and mitral valve(MV).
ATRIAL ANEURYSM.
A bulging or ballooning out of part of the wall of one of
the heart's upper chambers (atria ). If the aneurysm is present
in the wall between the atria (the atrial septum), it is also
known as an atrial septal aneurysm (ASA), an aneurysm of septum
primum or an aneurysm of the septum secundum.
An ASA has been associated with an increased risk of stroke
and is often accompanied by the presence of a patent foramen
ovale (PFO), an opening between the upper chambers (atria) of
the heart. Normally, this opening is present in the developing
fetus, and closes shortly after birth. It is often present since
birth (congenital).
Atrial Septal Aneurysm
Banu Mahalingam, M.D.,Echocardiography Journal:http://www2.umdnj.edu/%7Eshindler/ias2la.gif
Atrial Septal Aneurysm: case presentation at the Froedtert
Hospital Grand Rounds web site.
Echocardiographic Classifications of Atrial Septal Aneurysm
Motion
J Am Soc Echocardiogr 1997;10:644-56.
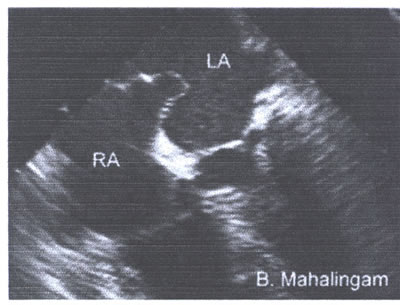
asaFig.22a-fig6.jpg :Atrial septal aneurysm protrudes from
the midline of the atrial septum to the left atrium .
Type 1R: The ASA protrudes from the midline of the atria to
the right atrium throughout the cardiorespiratory cycle.
Type 2L: The ASA protrudes from the midline of the atrial septum
to the left atrium throughout the cardiorespiratory cycle.
Type 3RL: The maximal excursion of the ASA is toward the right
atrium with a lesser excursion toward the left atrium.
Type 4LR: The maximal excursion of the ASA is toward the left
atrium with a lesser excursion toward the right atrium.
Type 5: The ASA movement is bidirectional and equidistant to
the right as well as to the left atrium during the cardiorespiratory
cycle.
Am J Cardiol 1985;56:653-67.
Type 1: The ASA projects into the right atrium during diastole,
with early systolic bulging into the left atrium, followed by
a rightward crossing-over motion in mid-systole and during inspiration
or expiration.
Type 2: Sustained rightward deviation during expiration and
a leftward motion only during inspiration in early ventricular
systole.
Type 3: The ASA remains in the right atrium, with an undulating
motion during all phases of the cardiorespiratory cycle.
J Am Coll Cardiol 1985;6:1370-82.
Type 1A: The bulging in the right atrium is motionless.
Type 1B: The bulging is confined to the right atrium but with
rapid phasic oscillation during inspiration.
Type 2: The ASA protrudes maximally into the left atrium and
is accompanied by excursion into the right atrium.
Association with Cerebrovascular Events:
Low incidence of embolic strokes with atrial septal aneurysms:
A prospective, long-term study
Burger AJ; Sherman HB; Charlamb MJ
Am Heart J 2000 Jan;139(1 Pt 1):149-52
Abstract
BACKGROUND:
Previous retrospective studies have suggested that atrial septal
aneurysms (ASA) are associated with embolic strokes. The purpose
of this study was to evaluate prospectively the embolic potential
of ASA. METHODS: Of 846 consecutive patients undergoing cardiac
surgery from December 1990 to March 1993, we identified 42 patients
who had ASA as an incidental finding on intraoperative transesophageal
echocardiography. Patency was determined by color and/or contrast
echocardiography. The majority of patients were given aspirin
postoperatively. Patients were monitored by personal and/or
telephone interviews, and their clinical conditions were confirmed
by their personal physicians. Any patient with any question
of a neurologic event had a detailed neurologic history, examination,
and computed tomographic or magnetic resonance imaging scan.
RESULTS: The incidence of ASA in our population was 4.9%; there
were 22 men and 20 women with a mean age of 72 years. Oscillating
ASA were present in 28 patients and fixed aneurysm in 10. The
mean diameter of the ASA was 21 +/- 4 mm. Eighteen (56%) of
32 patients had a patent ASA. Patients were monitored for a
mean period of 69.5 months (56 to 85 months). No patient had
a cerebrovascular event or systemic embolization.
CONCLUSION:
The risk of cerebrovascular events or embolic strokes in our
patient population with incidental ASA was low. If treatment
is needed for this condition, aspirin appears to be effective
therapy.
Mugge A. Daniel WG. Angermann C. Spes C. Khandheria BK. Kronzon
I. Freedberg RS. Keren A. Denning K. Engberding R. et al.
Atrial septal aneurysm in adult patients. A multicenter study
using transthoracic and transesophageal echocardiography
Circulation. 91(11):2785-92, 1995 Jun 1.
Abstract
BACKGROUND:
An atrial septal aneurysm (ASA) is a well-recognized abnormality
of uncertain clinical relevance. We reevaluated the clinical
significance of ASA in a large series of patients. The aims
of the study were to define morphological characteristics of
ASA by transesophageal echocardiography (TEE), to define the
incidence of ASA-associated abnormalities, and to investigate
whether certain morphological characteristics of ASA are different
in patients with and without previous events compatible with
cardiogenic embolism.
METHODS AND RESULTS:
Patients with ASA were enrolled from 11 centers between May
1989 and October 1993. All patients had to undergo transthoracic
and transesophageal echocardiography within 24 hours of each
other; ASA was defined as a protrusion of the aneurysm >
10 mm beyond the plane of the atrial septum as measured by TEE.
Patients with mitral stenosis or prosthesis or after cardiothoracic
surgery involving the atrial septum were excluded. Based on
these criteria, 195 patients 54.6 +/- 16.0 years old (mean +/-
SD) were included in this study. Whereas TEE could visualize
the region of the atrial septum and therefore diagnose ASA in
all patients, ASA defined by TEE was missed by transthoracic
echocardiography in 92 patients (47%). As judged from TEE, ASA
involved the entire septum in 100 patients (51%) and was limited
to the fossa ovalis in 95 (49%). ASA was an isolated structural
defect in 62 patients (32%). In 106 patients (54%), ASA was
associated with interatrial shunting (atrial septal defect,
n = 38; patent foramen ovale, n = 65; sinus venosus defect,
n = 3). In only 2 patients (1%), thrombi attached to the region
of the ASA were noted. Prior clinical events compatible with
cardiogenic embolism were associated with 87 patients (44%)
with ASA; in 21 patients (24%) with prior presumed cardiogenic
embolism, no other potential cardiac sources of embolism were
present. Length of ASA, extent of bulging, and incidence of
spontaneous oscillations were similar in patients with and without
previous cardiogenic embolism; however, associated abnormalities
such as atrial shunts were significantly more frequent in patients
with possible embolism.
CONCLUSIONS:
As shown previously, TEE is superior to the transthoracic
approach in the diagnosis of ASA. The most common abnormalities
associated with ASA are interatrial shunts, in particular patent
foramen ovale. In this retrospective study, patients with ASA
(especially with shunts) showed a high frequency of previous
clinical events compatible with cardiogenic embolism; in a significant
subgroup of patients, ASA appears to be the only source of embolism,
as judged by TEE. Our data are consistent with the view that
ASA is a risk factor for cardiogenic embolism, but thrombi attached
to ASA as detected by TEE are apparently rare.
Cabanes L. Mas JL. Cohen A. Amarenco P. Cabanes PA. Oubary
P. Chedru F. Guerin F. Bousser MG. de Recondo J.
Atrial septal aneurysm and patent foramen ovale as risk factors
for cryptogenic stroke in patients less than 55 years of age.
A study using transesophageal echocardiography.
Stroke. 24(12):1865-73, 1993 Dec.
Abstract
BACKGROUND AND PURPOSE:
An association between atrial septal aneurysm and embolic events
has been suggested. Atrial septal aneurysm has been shown to
be associated with patent foramen ovale and, in some reports,
with mitral valve prolapse. These two latter cardiac disorders
have been identified as potential risk factors for ischemic
stroke. The aim of this prospective study was to assess the
role of atrial septal aneurysm as an independent risk factor
for stroke, especially for cryptogenic stroke.
METHODS:
We studied the prevalence of atrial septal aneurysm, patent
foramen ovale, and mitral valve prolapse in 100 consecutive
patients < 55 years of age with ischemic stroke who underwent
extensive etiological investigations. We compared these results
with those in a control group of 50 consecutive patients. The
diagnosis of atrial septal aneurysm and patent foramen ovale
relied on transesophageal echocardiography with a contrast study
and that of mitral valve prolapse, on two-dimensional transthoracic
echocardiography. RESULTS: Stepwise logistic regression analysis
showed that atrial septal aneurysm (odds ratio, 4.3; 95% confidence
interval, 1.3 to 14.6; P = .01) and patent foramen ovale (odds
ratio, 3.9; 95% confidence interval, 1.5 to 10; P = .003) but
not mitral valve prolapse were significantly associated with
the diagnosis of cryptogenic stroke. The stroke odds of a patient
with both atrial septal aneurysm and patent foramen ovale were
33.3 times (95% confidence interval, 4.1 to 270) the stroke
odds of a patient with neither of these cardiac disorders. For
a patient with atrial septal aneurysm of > 10-mm excursion,
the stroke odds were approximately 8 times the stroke odds of
a patient with atrial septal aneurysm of < 10 mm.
CONCLUSIONS:
This study shows that atrial septal aneurysm and patent foramen
ovale are both significantly associated with cryptogenic stroke
and that their association has a marked synergistic effect.
Atrial septal aneurysms of > 10-mm excursion are associated
with a higher risk of stroke.
Pearson AC. Nagelhout D. Castello R. Gomez CR. Labovitz AJ.
Atrial septal aneurysm and stroke: a transesophageal echocardiographic
study.
Journal of the American College of Cardiology. 18(5):1223-9,
1991 Nov 1.
Abstract
The prevalence and morphologic characteristics of atrial septal
aneurysms identified by transesophageal echocardiography in
410 consecutive patients are described. Two groups of patients
were compared: Group I consisted of 133 patients referred for
evaluation of the potential source of an embolus and Group II
consisted of 277 patients referred for other reasons. An atrial
septal aneurysm was diagnosed by transesophageal echocardiography
in 32 (8%) of the 410 patients. Surface echocardiography identified
only 12 of these aneurysms. Atrial septal aneurysm was significantly
more common in patients with stroke (20 [15%] of 133 vs. 12
[4%] of 277) (p less than 0.05); right to left shunting at the
atrial level was demonstrated in 70% of patients in Group I
and 75% of patients in Group II by saline contrast echocardiography.
Four patients in Group I had an atrial septal defect with additional
left to right flow. There was no difference between the two
groups in aneurysm base width, total excursion or left atrial
or right atrial excursion. However, Group I patients had a thinner
atrial septal aneurysm than did Group II patients.
It is concluded that an atrial septal aneurysm occurs commonly
in patients with unexplained stroke, is more frequently detected
by transesophageal echocardiography than by surface echocardiography
and is usually associated with right to left atrial shunting.
Treatment (anticoagulant therapy vs. surgery) of atrial septal
aneurysm identified in stroke patients can be determined only
by long-term follow-up studies.
Belkin RN., Hurwitz BJ., Kisslo J.
Atrial septal aneurysm: association with cerebrovascular and
peripheral embolic events.
Stroke. 18(5):856-62, 1987 Sep-Oct.
Abstract
Patient records in 36 consecutively identified patients with
typical echocardiographic findings of atrial septal aneurysm
were reviewed. Ten of the 36 (28%) had cerebrovascular events.
Of these 10, 5 had completed strokes of definite embolic origin
on the basis of clinical, angiographic, and computed tomographic
findings; 2 had transient ischemic attacks of probable embolic
origin. One of the 36 patients had a definite peripheral vascular
embolus. Thus, 6 of 36 consecutively identified patients with
atrial septal aneurysm (17%) had definite embolic events and
8 of 36 (22%) had definite or possible embolic events.
The cause of the association between atrial septal aneurysm
and emboli is unknown. While aneurysm-associated thrombus has
been suggested, the high proportion (90%) of patients with interatrial
shunting demonstrated by contrast echocardiography in this study
suggests paradoxical embolization as a potential cause. Whatever
its mechanism, the high prevalence of embolic events in this
series strongly supports the premise that atrial septal aneurysm
is a cardiac abnormality with embolic potential.
JANUARY 2002: Contents
Clin. Cardiol. 25,42 (2002)
Images in Cardiology
This section edited by Edward A. Geiser, M.D.
A Jumping Rope Giant Atrial Septal Aneurysm and Territorial
Disputes
Ara Sadaniantz, M.D., FACC, FESC
The Miriam Hospital, Brown University School of Medicine, Providence,
Rhode Island. USA
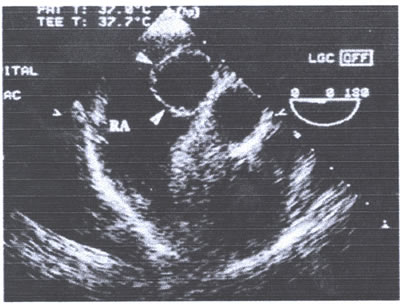
asaFIG22b-fig7.jpg:In the short-axis view a spherical cystic-appearing
mass (arrows) measures 2 cm in diameter. Arrows = septum secundum,
RA = right atrium.
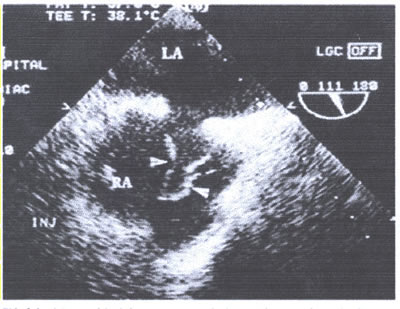
asaFIG22c-fig8.Base of ostium secundum measured 1.2cm.The menbrane
had the appearance of a jumping rope,protruding 3.7cm into the
right atrium with phasic excursion, but it did not enter the
left atrium(LA).
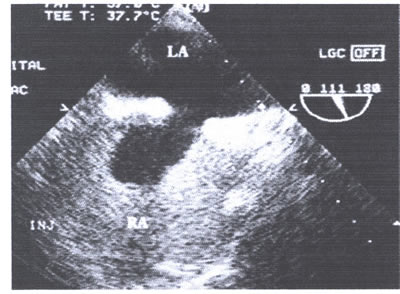
asaFIG22d-fig.9.jpg: Agitated saline contrast injection into
an antecubital vein opacifies the right atrium without any evidence
of left to right shunting. Abbreviations as in asa figures 7and
8.
Interatrial shunt is the most common abnormality associated
with atrial septal aneurysm (ASA) and its detection is more
common with transesophageal echocardiogram. The definition of
ASA includes protrusion of atrial septum 15 mm or greater beyond
the plane of interatrial septum with phasic excursion during
cardiorespiratory cycle.
An 81-year-old woman was referred for transesophageal echocardiogram
to further evaluate a possible mass in the right atrium that
appeared to be suspicious on transthoracic. echocardiogram.
She was sedated with 1 mg Versed® and 25 mg Demerol(R) IV
and her oropharynx was anesthetized with xylocaine spray. The
probe was introduced without difficulty into the esophagus.
In the short-axis view a spherical cystic-appearing mass measuring
2 cm in diameter was identified (asaFig22b-fig.7.jpg). In the
long-axis view visualizing the atrial septum, an extensively
redundant septum secundum membrane was identified overlaying
the ostium secundum. The base of ostium secundum measured 1.2
cm (asaFig22c-fig:8). The membrane, when seen in real time,
had the appearance of a jumping rope. It protruded 3.7 cm into
the right atrium with phasic excursion but it did not enter
the left atrium. An agitated saline solution that filled the
right atrium without any evidence of left to right shunting
was used as contrast agent. The negative contrast in the right
atrium, beyond the ostium secundum, is functionally within the
territory of the left atrium due to the giant atrial septal
aneurysm (asasFig. 22d-fig:9).
In conclusion, what is justly the right atrial territory is
utilized by and functions as the left atrium.
Reference
1. Agmon Y, Khandheria BK, Meissner I, Gentile F, Whisnant .JP,
Sicks JD, O'Fallon WhM, Covalt JL. Wiebers DO, Seward JB: Frequency
of atrial septal aneurysms in patients with cerebral ischemic
events. Circulation 1999;99:1942-1944
Atrial Septal Aneurysm: A Study in Five Hundred Adult Patients
Olivares-Reyes Alexander; Al-Kamme Ahmad; Gonzalez JavierCardiology
Department The Brooklyn Hospital Brooklyn, New York, USA
Abstract
Introduction:
Today, atrial septal aneurysm (ASA) is well recognized pathology,
characterized as a «saccularv deformity, generally at
the level of the fossa ovale, which protrudes to the right or
left atrium or both. This entity is strongly associated to cardioembolic
events as well as other acquired and congenital heart diseases.
Material and Methods: Over a six and one half year period we
have prospectively studied clinically and echocardiographically
(including transesophageal echocardiogram (TEE) in 200 (40%
) patients), 500 consecutive adults from a total of 22,224 patients
(pts) with the diagnosis of ASA.
Results:
We found a prevalence of 2.2%, a mean age of 65 yr. , 317 (63%)
women and 183 (37%) men. Clinical associations included, HTN
64%, pulmonary HTN 36%, cerebrovascular events (CVE) 23%, Diabetes
23%, heart failure 16%, atrial arrhythmias 13%, COPD 7%, and
chronic renal failure 5%. Echocardiographically: valvular abnormality
86% vs 14% without, left ventricular hypertrophy 44%, PFO 36%
(40/110), LA enlargement (LAE) 31%, valvuiar prolapse 13%, RAE
11%, LVE 10%, RVE 8%, atrial septal defect 7 %, vegetations
2.4%, and thrombi 1.2%. The types of ASA were: 1R 16%, 2L 31%,
3RL 12%, 4LR 33%, and type 5, 8% (according to our previously
published ASA classification). Mobile ASA 54%, fixed ASA 46%.
Left bulging predominance 69% vs 31% right bulging predominance.
Type 5 was excluded. Interesting data were found when we analyzed
and compared variables like age, gender, type of ASA as well
as other concomitant heart diseases. In pts with pulmonary HTN,
predominantly left bulging ASA were 114 (69%) vs 52 (31%)* right
bulging; COPD 27 (84%) vs 5 (16%)*. In pts with atrial septal
defect, predominantly right bulging, 25 (78%) vs 9 (22%)*, while
patent foramen ovale pts had left bulging 28 (80%) vs 7 (20%)*
of right bulging. (* p <0.05).
Conclusions:
This study supports the commonality in identifying ASA by transthorasic
as well as transesophageal echocardiogram. Its definitive association
with acquired as well as congenital heart diseases, but also
as an isolated and totally asymptomatic entity. Its frequent
correlation with cerebrovascular embolic events. It is more
prevalent in female, left atrial bulging types of ASA, and a
tendency towards the mobile types of ASA.
Introduction
Atrial Septal Aneurysm (ASA) is a localized c<saccular>>
deformity of the interatrial septum (IAS), generally at the
level of the fossa ovale, which bulges into the right or left
atrium or both. ASA was initially thought to be a rare congenital
abnormality, however, with the advent of two-dimensional echocardiography
and more recently, the widespread use of transesophageal echocardiography
(TEE) it has become more easily and more frequently identified
in patients.
Prevalence.
The prevalence of ASA varies, but transthoracic echocardiographic
(TTE) studies estimate the rate to be between 0.08% and 1.2%.
In a large autopsy series the prevalence reported was 1 %. More
recent studies using TEE have demonstrated a prevalence between
2% and 10%. In the pediatric patient population the prevalence
reported by TTE is 0.9% to 1.7% in children and 4.9% in infants
.
ASA association.
Atrial septa) aneurysm has been associated with congenital
heart diseases such as patent foramen ovale (PFO), atrial septal
defects (ASD), ventricular septa) defects (VSD), valvular prolapse
(VP), patent ductus arteriosus (PDA), Ebstein's anomaly, and
tricuspid and pulmonary atresia as well as acquired heart diseases
including valvular disease, cardiomyopathy, systemic and pulmonary
hypertension, ischemic heart disease, arrhythmias and thrombus
formation. More recently a number of studies found an association
between ASA and cerebrovascular events (CVE) of embolic origin,
including transient ischemic attacks (TIA) and cerebrovascular
accidents (CVA).
Objectives
The main objective of this study is to analyze and correlate
the clinical and echocardiographic characteristics of patients
with this, everyday more diagnosed, cardiac abnormality.
Material and Methods
During the period of January 1991 and June 1997, we studied
22,224 patients which were referred for transthoracic echocardiography.
Of these, 500 patients fulfilled the echocardiographic criteria
for atria) septal aneurysm.
Echocardiographic examination.
The echocardiographic studies were performed using three commercially
available ultrasound systems (Acuson 128, Acuson 128 XP/10c,
and Hewlett-Packard sonos 500) with 2.5 to 4 MHz phased array
imaging transducers. All systems were capable of both Doppler
color and spectral flow. All patients underwent standard TTE
views including parasternal long axis, short axis, apical five,
four, three, and two chamber views as well as subcostal four
chamber and short axis views. The studies were performed with
the patient in supine and left lateral decubitus positions during
quiet respiration. Particular attention was given to subcostal
views with appropriate transducer angulation to visualize the
heart completely.
In four chamber view and the interatrial septum with its foramen
ovale segment in particular. The atria, including the atrioventricular
valves, was magnified to ease the visualization of movement
and measurement of the ASA. Patients were placed in the supine
position with legs and knees flexed. They were in quiet respiration
and sustained inspiration.
Transesophageal echocardiogram was performed in 200 patients
with prior TTE studies and who had a diagnosis or suspicion
of ASA. All of them had additional indications such as rule-out
source of embolism, masses or thrombus, intracavitary shunts,
vegetations, etc. TEE was performed after administration of
oropharyngeal anesthesia with lidocaine (10%) or aerosolized
benzocaine (14%), and occasionally diazepam for sedation. The
studies were performed using an Acuson 128 and Acuson 128 XP/10c
ultrasound system, with 5-7 MHz single or bi-plane TEE probes.
Standard TEE views were obtained.
Contrast study.
Contrast studies were performed during the TEE in patients
in whom intracavitary shunt was suspected.
Criteria for atrial septal aneurysm.
The diagnostic criteria for ASA was made if a sacculation or
deformity in the interatrial septum or the foramen ovale region
was seen. An excursion of = 10 mm into the right or left atrium
or if the sum of bilateral excursions of > 10 mm was required.
The minimal aneurysmal base amplitude (width) accepted in this
study was 15 mm in diameter. The aneurysm was observed in subcostal
view, apical four chamber, and parasternal short axis views
at the level of the great vessels. Sometimes the bulging was
also seen in apical two and three chamber views. The classification
of ASA was made according to its different movements, as in
previous classifications and regardless of its possibly different
etiology. All measurements done in this study were made according
to the recommendations of the American Society of Echocardiography.
All the studies were taped and hard copies were taken for further
analysis and measurements. All cases were reviewed by three
different observers.
Statistical analysis. The data were analyzed with Student's
t test and chi-square test and are given as mean +- standard
deviation. A p value <0.05 was considered significant.
Results
We found an ASA prevalence of 2.2% (500 ASA in 22,224 patients
studied in our laboratory during a period of 6 + years.The mean
age was 65 years.A predominance in women with 317 Pts.(63%).See
Table1(asafig22e-fig10.jpg)below:
-fig10.jpg)
All patients with ASA were studied and classified in one of
five types of ASA, according to a new classification previously
published by us. asaFig22f -fig:11 describes the new classification,
while asaFig22g-fig:12 shows the numbers and percentages of
patients with the different types of ASA, Patients with left
bulging predominance represented the majority with a 64% of
the total population and a 69% if compared with the predominantly
right bulging type of ASA.
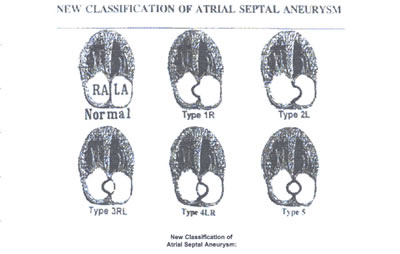
asaFig22f -fig:11:New Classification of Atrial Septal Aneurysm:
Echocardiographic four-chamber view,as well as the different
bulgings of the atrial septum with aneurysm, showing the new
classification of atrial septal aneurysm and how normally the
atrial septum is seen in a two-dimensional depiction.
TYPE 1R: The ASA protrudes from the midline of the atrial to
the right atrium throughout the cardiorespiratory cycle.
TYPE 2L: The ASA protrudes from the midline of the atrial septum
to de left atrium throughout the cardiorespiratory cycle.
TYPE 3RL: The maximal excursion of the ASA is toward the right
atrium with a lesser excursion toward the left atrium.
TYPE 4LR:The maximal excursion of the ASA is toward the left
atrium with a lesser excursion toward the right atrium.toward
the left atrium with a lesser excursion toward the right atrium.
TYPE 5:The ASA movement is bidirectional and equidistant to
the right as well as to the left atrium during the cardiorespiratory
cycle.
-fig13.jpg)
asaFig22h-fig13:Clinical and echocardiographic variables are
described in Table 2 above.
In this study we also analyzed the mobility of the ASA, and
we divide them into 2 groups: the <fixed >ASA (types 1R
and 2L, which only bulge within an atrium) 231 Pts. (46%), and
the <mobile> ASA (types 3RL, 4LR, and Type-5, which bulge
bidirectionally into both atria), 269 Pts. (54%),asaFig22h-fig13.
-fig14.jpg)
A highlight of these variables are represented in asaFig22i-fig14.
In patients with pulmonary HTN, predominantly left bulging ASA
were 114 (69%) vs 52 (31 %) right bulging; COPD 27 (84%) vs
5 (16%); PFO 28 (80%) vs 7 (20%). In the other hand, patients
with ASD had predominantly right bulging in 25 (78%) vs 9 (22%)of
left bulging. ('p< 0.05).
Discussion
ASA is becoming more prominent in clinical cardiology. Its association
with cardiac abnormalities, in particular cardiogenic embolic
events, contributes to its significance. Only about 100 cases
of ASA had been reported before 1985; however, in the last 10
years, and because of new and better 2D echo machines and TEE,
a considerable number of cases has been published. To date,
this is the largest casuistic study of patients with ASA. The
association of ASA with clinical variables like CVE, HTN, CAD,
DM, valvular prolapses, arrhythmias, valvulopathies, PFO, ASD,
etc., is identified in this large group of patients like can
be observed in Table 2.
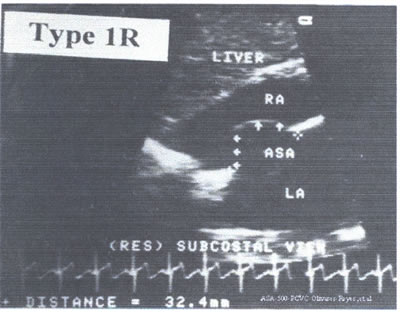
An Echo with an ASA type 1 R is represented in asafig22j-fig15.jpg.
This is an amplified 4C subcostal view of a 65 yr old lady with
history of CHF and moderate mitral regurgitation.
Conclusions
From this study we can conclude: 1) the commonality in identifying
ASA by TTE and TEE, 2) Its association with acquired as well
as congenital heart diseases, 3) that there is a group of patients
with isolated and asymptomatic ASA, 4) Its frequent association
to stroke, 5) the left predominance type and mobile ASA., and
6) the tendency to be more frequent in female patients.
References
1. Olivares-Reyes A, et at. Atrial Septal Aneurysm: A new classification
in 205 adults. J Am Soc Echocardiogr 1997:10:644-56.
2. Silver MD, Dorsey JS. Aneurysm of the septum primum in adults.
Arch Pathol Lab Med 1978;102:62-5.
3. Henley PC et al. Diagnosis and classification of atrial septa)
aneurysm by two-dimensional echocardiography: report of 80 consecutive
cases. J Am Coll Cardiol 1985;6:1370-82.
4. Longhini C, et al. Atdal septet aneurysm: echocardiographic
study. Am J Cardiol 1985;56:653-67,
5. Mugge A, et al. Atrial septet aneurysm in adult patientsi
a multicenter study using transthoracic and transesophageal
echocardiography. Circulation 1995;19:2785-92.
6. Hauser AM, et al. Aneurysm of the atria) septum as diagnosed
by echocardiography: analysis of 11 patients. Am J Cardiol 1984;53:1401-2.
7. Gondi B, Nanda NC. Two-dimensional echocardiographic features
of atria) septa aneurysm. Circulation 1981,63:452-57
2)
Cyanotic Congenital Heart Disease
This
group features bluish discoloration of the skin and lips as
opposed to the normal pink appearance. The cyanosis is due to
the shunting of systemic venous blood to the arterial circulation
causing arterial blood desaturation of oxygen.
The size of the shunt determines the degree of desaturation.
In adults the most common causes of cyanotic congenital heart
disease are tertralogy of Fallot and Eisenmenger's syndrome.
1.Tetralogy of Fallot
It
is characterized by a large ventricular septal deftect (VSD),
an aorta that overrides the left and right ventricles, obstruction
of the right ventricular (RV) outflow tract, and RV hypertrophy
(increased wall thickness).
As obstruction in RV outflow tract increases, more blood is
shunted through the VSD to the left side of the heart to cause
more cyanosis (see figure
23c).
Increases
in resistance to flow in the general arteries of the body causes
less shunting, and decreases cause more shunting to the left.
Symptoms
in adults include shortness of breath and limited exercise tolerance.
Complications include brain abscesses, strokes and heart infections.
Such patients may have enlargement of the distal ends of their
fingers called clubbing.
Most patients without surgical correction die in childhood.
Echocardiography
can establish the diagnosis. Color Doppler can visualize the
VSD.
Heat catherterization can confirm the diagnosis.
Surgical
repair is recommended to relieve symptoms and to improve survival.
Complete surgical correction (closure of the VSD and relief
of RV outflow obstruction is performed currently when patients
are very young.
Patients are at risk for heart infections and should thus receive
prevention with antibiotics before dental or elective surgical
procedures.
Even
with repair these patients have a poorer survival rate (apparently
due to cardiac causes such as arrhythmias).
2.
Ebstein's Anomaly
This
anomaly is due to a defect in the tricuspid valve (TV) with
the septal and posterior leaflets displaced down into the right
ventricle, while the anterior leaflet is malformed and abnormally
attached to the RV free wall (see figure
23d).
This valve often allows blood to regurgitate from the small
RV back into the large RA.
Eighty percent of these patients have ASD's through which right-to-left
shunting of blood may occur with cyanosis.
Such patients are at risk for a paradoxical embolus (blood clot)
from the RA through the LA to the brain with abscess, and sudden
death.
There
is usually a heart murmur.
EKG
abnormalities are often present including WPW syndrome (see
figure
1, figure
2 and figure
3).
Twenty percent have an accessory electrical pathway between
the atrium and ventricle (see figure
1).
Echocardiogram
can define the abnormalities, and a color Doppler imaging can
determine the presence and size of interatrial shunting.
Management
involves prevention of complications, such as heart infection,
prevented with antibiotic prophylaxis.
Heart
failure is treated with diuretics (diuril, lasix, etc) (to eliminate
fluid) and digoxin (a heart drug to improve heart muscle contractions).
Arrhythmias may be treated with medication or catheter ablation
(see figure
11).
Repair
or replacement of TV in conjunction with closure of the interatrial
communication is recommended in older patients with severe symptoms
despite medical therapy and heart enlargement.
3.
Transposition of the Great Arteries
In
d-transposition of the great arteries, the aorta arises in an
anterior position from RV and the pulmonary artery arises from
LV (see figure
23e).
In two thirds of cases the ductus arteriosus (see figure
22) and foramen ovale allow communication between the aortic
and pulmonary circulations.
Severe cyanosis is present.
The one third with other defects that permit intracardiac mixing
(i.e. ASD figure 20, VSD figure
21, PDA) are less critically ill with loss severe cyanosis,
but they are at risk of LV failure.
Findings
include cyanosis and heart murmur.
RVH (RV wall enlargement) or LVH (LV wall enlargement) may be
present. Chest X ray shows heart enlargement.
Immediate
management involves creating intracardiac mixing or increasing
its extent:
1) use of infusing of medication, prostaglandine E to maintain
or restore patency of ductus arterioses, the creation of an
ASD or both.
Also, oxygen is administered to most patients (to decrease pulmonary
[lung] vascular (blood vessel) resistance and to increase lung
blood flow), as are digoxin and diuretic drugs like diuril or
lasix (to treat heart failure).
 Figure
23e
Figure
23e
Two
surgical operations have been used (see figure 23e regarding
the atrial switch operation).
The atrial switch operation as shown in figure 23e has been
replaced by the arterial switch operation in which the pulmonary
artery and ascending aorta are transected above the semilunar
valves and coronary arteries (see figure 23e), and then switched,
so that the aorta is connected to the neoaortic valve (formerly
the pulmonary valve) arising from the left ventricle (LV), and
the pulmonary artery is connected to the neopulmonary valve
(formerly the aorta valve) arising from the RV (see figure 23e).
The coronary arteries are relocated to the neoaorta to restore
normal coronary circulation.
This
operation can be performed in neonates (newly born) and is associated
with a low operative mortality and an excellent long-term outcome.
4.
Esenmenger's Syndrome
This
consist of a large left (L) to right (R) shunt, which causes
severe pulmonary (lung) vascular disease and high blood pressure
(in the lungs) with resulting reversal of the direction of shunting
( figure
23f ).
This shunting with increase pressure causes the lung arteries
to narrow due to thickening of their walls (especially the middle
wall, called tunica media, see figure
23f) and cause obstruction.
Initially
the changes may be reversible, but ultimately they become irreversible
due to inflammation of the arteries.
Hence, much of the lung arteries are occluded, leading to increase
pulmonary blood vessel resistance. Ultimately the resistance
in the lungs may exceed the resistance in the arteries of the
rest of the body, which leads to a reversal of flow from left-to-right
to right-to-left shunt.
The
reversal of the shunt leads to cyanosis, as well as shortness
of breath, coughing up blood, reduced exercise tolerance, syncope
(fainting), palpitations, and atrial fibrillation (see figure
15a, figure
15b).
Brain events such paradoxical embolus, thrombosis (stroke) and
hemorrhage may occur.
Heart failure suggest a poor prognosis, and sudden death is
possible.
Digital
swelling (clubbing) may occur. Heart murmurs may occur.
EKG
may show RVH and atrial arrhythmias (see figure
2, figure
3, figure
4 and figure
5).
Echocardiogram
shows RV pressure overload, pulmonary high blood pressure, and
the underlying heart defect.
Using intravenous contrast injections along with echocardiogram
will visualize the intracardiac defect. Heart catherterization
is necessary to assess the lung hypertension and the size of
the defect.
Rate
of survival is 80% 10 years after diagnosis, 77% at 15 years,
and 42% at 25 years. Death is usually sudden, presumably due
to arrhythmias, but some die of the above mentioned complications.
Lung
transplantation with repair of the cardiac or combined heart-lung
transplantation is an option for patients with a poor prognosis
(failing to respond to medical therapy).