Anomalies
of the Great Veins
Due
to major advances in both diagnosis and treatment of congenital
heart disease in children, many are living into adulthood. There
are almost one million such patients this year.
Congenital
heart disease can be divided into two types:
1.
The acyanotic ones in which the oxygen level in the blood is
high enough to keep the patients' color pink;
2.
The cyanotic ones in which the oxygen level in the blood is
low enough for the lips and skin to show varying degrees of
bluish discoloration.
1.
Acyanotic congenital heart consist of the following:
a)
atrial septal defect
( figures 112a,
112b
)
b) ventricular septal
defect ( figure 112c
)
c) patent
ductus arteriosus ( figure 22
)
d) aortic stenosis ( figures
24a,
24b,
46a,
46b,
46c,
47
)
e) pulmonary stenosis
( figure 25a,
25b
)
f) parachute
mitral valve ( figures 44g-1
and 44g-2
)
g)
coronary artery fistula
h) anomalies of the great veins
2.
Cyanotic Congenital Heart
Disease features bluish
discoloration of the skin and lips as opposed to the normal
pink appearance. The cyanosis is due to the shunting of systemic
venous blood to the arterial circulation causing arterial
blood desaturation of oxygen. The size of the shunt determines
the degree of desaturation. In adults the most common causes
of cyanotic congenital heart disease are tetralogy of Fallot
and Eisenmenger's syndrome.
a) Tetralogy
of Fallot ( figure 23d
)
b) Ebstein's Anomaly
( figure 23e
)
c) Transposition
of the Great Arteries ( figure 23h
)
d ) Eisenmenger's syndrome
( figure 23j
)
Brickner,M.E.
and others,Congenital Heart Disease in Adults,N.Engl.J.Med.,Vol.342.N4,2000,pp.334-342
Anomalies of the Great Veins
--------------------------------------------------------------------------------
Introduction
Anomalies of the Systemic Veins
Anomalies of the Pulmonary Veins
Anomalies of the Coronary Sinus
--------------------------------------------------------------------------------
Introduction
In the normal heart, the superior and
inferior vena cavea, along with the coronary sinus, have a characteristic
arrangement within the right atrium. It is therefore appropriate
to consider abnormalities of each channel separately, recognizing
that, in rare cases, these anomalies may coexist.
Anomalies of the systemic veins are not uncommon,
examples of which include a persistent left superior vena cava
connected to the coronary sinus, interrupted inferior vena cava,
and absent right superior vena cava.
Anomalies of the systemic veins are associated
with atrial isomerism, an understanding of which is important
in sorting out the various lesions involved. These so-called
heterotaxic syndromes are characterized by failure of many "right-left"
differentiation, leading to ambiguity in viscero-atrial situs,
along with anomalies of systemic or pulmonary venous return.
In patients with left atrial isomerism, the
infrahepatic portion of the inferior vena cava is frequently
absent, and the venous return from the lower part of the body
enters the superior vena cava via the azygos vein. In patients
with right atrial isomerism, the right and left hepatic veins
may enter the ipsilateral sides of the common atrium, remaining
separate from the inferior vena caval entrance.
Abnormalities of the pulmonary veins are also
common in both left and right atrial isomerism; direct connection
to the superior or inferior vena cava is more frequent in right
atrial isomerism, whereas anomalous pulmonary venous drainage
into the same side of the atrium as the systemic venous drainage
is more frequent in left atrial isomerism. Frequently there
is outflow obstruction to pulmonary arterial blood flow at either
the valvar or subvalvar level. Pulmonary atresia is more common
with right atrial isomerism, whereas pulmonary stenosis is more
common in left atrial isomerism. Pulmonary artery anomalies
are not rare, particularly when there is pulmonary atresia with
the ductus arteriosus as the only source of pulmonary blood
flow. After the ductus closes, a "coarctation" commonly
develops in the pulmonary artery just at the insertion of the
ductus. The branching pattern of the pulmonary arteries generally
assumes one of two forms, depending on whether left or right
atrial isomerism is present.
In right atrial isomerism, both right and
left pulmonary arteries tend to look like a normal right pulmonary
artery, with the bronchus for the upper lobe being above the
first segmental artery for the right upper lobe (eparterial
bronchus).
In contrast, in left atrial isomerism, the
bronchi are below the pulmonary artery at the hilum (hyparterial
bronchi), as is the case for a normal left pulmonary artery.
In right atrial isomerism, both lungs tend to be trilobed, whereas
in left atrial isomerism both lungs tend to be bilobed.
Finally, asplenia is more commonly present
in right atrial isomerism, whereas polysplenia is more frequently
associated with left atrial isomerism. These features have contributed
to the general rule (which has many exceptions) that patients
with right atrial isomerism tend to have bilateral "right-sidedness",
whereas those with left atrial isomerism tend to have bilateral
"left-sidedness".
--------------------------------------------------------------------------------
Anomalies of the Systemic Veins
This discussion in limited to anomalies of the great systemic
veins, which include the superior and inferior vena cava, along
with the coronary sinus.
Anomalies of the superior vena cava
A persistent left superior
vena cava is the most common form of anomalous venous drainage
involving the superior vena cava and represents persistence
of the left horn of the embryonic sinus venosus, which normally
involutes during normal development to become the coronary sinus.
Almost always, a persistent left superior vena cava enters the
right atrium through the orifice of an enlarged coronary sinus.
To this extent, therefore, the lesion is considered to be an
anomaly of the coronary sinus. It characteristically reaches
the heart in the angle between the left atrial appendage and
the left pulmonary veins. The left superior vena cava then runs
down the back of the left atrium to enter the left atrioventricular
groove and channel draining blood from the head and both arms.
This is the site in the normal heart of the oblique ligament
and vein of Marshall.
A persistent left superior vena cava is of
no clinical significance since the systemic venous blood continues
to return to the right atrium, but may be troublesome in keeping
blood out of the field during cardiopulmonary bypass. It may
be ligated only in the presence of a communicating vein between
the right and left superior vena cavea. In some circumstances,
the left vena cava may be the only channel draining the head
and both arms, and the usual right superior vena cava is absent.
Rarely, a persistent left superior vena cava
can be connected to the roof of the left atrium between the
left atrial appendage and pulmonary veins rather than to the
coronary sinus. This anomaly is termed complete unroofing of
the coronary sinus. The orifice of the coronary sinus then persists
as an interatrial communication. A levo-atrial cardinal vein
is a rare venous structure that is found in association with
the hypoplastic left heart syndrome. It provides the only route
of exit for pulmonary venous return, and typically runs along
the roof of the left atrium, from the anticipated site of a
left superior vena cava, to the left brachiocephalic vein, and
the superior vena cava.
Other rare anomalies of the superior vena
cava include a right superior vena cava connected to left atrium,
and a right superior vena cava connecting with both the right
and left atria through separate orifices in the presence of
an intact atrial septum. Aneurysmal dilatation of the superior
vena cava is recognized as being an acquired lesion of the heart
and is rarely seen in children.
Anomalies of the inferior vena cava
Anomalies of the inferior vena cava are most
commonly an integral constituent of atrial isomerism, and only
rarely is found in patients with usual or mirror-image atrial
arrangements. The most common lesion of the inferior vena cava
is that of interruption of the abdominal portion, with continuation
through either the azygos or the hemiazygos veins. Described
simply as ‘azygos continuation’, it is important to always exclude
the existence of left atrial isomerism, which is performed through
the identification of the bronchial morphology and determination
of the presence of polysplenia. When there is interruption of
the inferior vena cava with azygos continuation, all the systemic
venous return reaches the morphologically right atrium through
a superior vena cava. With azygos continuation, this is the
right-sided vein, whereas with hemiazygos continuation, the
inferior caval blood is returned through a persistent left superior
vena cava.
Anomalies of the coronary sinus
Morphology
The most frequent morphological anomaly
of the coronary sinus is persistence of a left superior vena
cava which drains through the orifice of the coronary sinus.
Under these circumstances, the coronary sinus is enlarged, and
the lesion is of no clinical importance. An unroofed coronary
sinus, however, can produce windows into the left atrium and
provide right-to-left intraatrial shunting. The extreme form
of this lesion is completely unroofed coronary sinus, in which
the interatrial communication is at the mouth of the sinus.
Isolated coronary sinus windows can occur, however, when there
is no persistent left superior vena cava and when the atrial
septum is intact.
Other rare reported anomalies of the coronary
sinus include connection of hepatic veins to the coronary sinus,
fistulous connections between the coronary sinus and the coronary
arteries, and connection of the coronary sinus to the inferior
vena cava.
The unroofed coronary sinus syndrome consists
of total absence of the coronary sinus, as there is absence
of the partition between the coronary sinus and the left atrium.
Individual coronary veins drain separately into both the right
and left atria. The unroofed coronary sinus syndrome with persistent
left superior vena cava occurs when part or all of the common
wall between the coronary sinus and the left atrium is absent,
and there is a persistent left superior vena cava. The persistent
superior vena cava usually connects to the left upper corner
of the left atrium between the attachment of the left atrial
appendage and the left pulmonary veins.
There is often an associated coronary sinus
ASD, which may be further complicated by a confluent partial
or complete atrioventricular septal defect. Other associated
lesions include a patent foramen ovale, ostium secundum ASD,
tricuspid atresia, tetralogy of Fallot, and atrial isomerism.
Of considerable importance is that the innominate vein is absent
in the great majority of cases, and the right superior vena
cava is frequently small or absent. The inferior vena cava may
cross to the left side below the diaphragm and enters the left
hemiazygos vein, which subsequently drains into the left superior
vena cava. The hepatic veins usually enter the inferior aspect
of the right atrium, but they too may connect anomalously to
the inferior left atrial wall.
Hemodynamics
Isolated completely unroofed coronary
sinus is associated with a small right-to-left shunt and is
usually of no hemodynamic consequence. In the presence of a
persistent left superior vena cava, however, cyanosis may be
mild or severe depending on the degree of right-to-left shunting.
Clinical Findings & Management
Patients with completely unroofed coronary
sinus and persistent left superior vena cava present with cyanosis.
Cerebral embolization and cerebral abscess may also complicate
the clinical picture. The diagnosis of unroofed coronary sinus
syndrome is usually made by echocardiography. Cineangiography
may be useful in defining a persistent left superior vena cava
and/or inferior vena cava drainage to the left atrium, in addition
to defining commonly associated abnormalities.
Medical management is usually expectant, and
operative correction is usually indicated.
Isolated coronary sinus ASD (isolated unroofed
coronary sinus without persistent left superior vena cava) is
treated the same as other types of ASD. Unroofed coronary sinus
with persistent left superior vena cava is approached with the
goal of separating the systemic from pulmonary venous drainage.
The most direct method is to resect much of the atrial septum,
leaving a rim of limbus to preserve the conduction system, then
separate the three systemic veins from the four pulmonary veins
by means of a pericardial patch. Left superior vena cava ligation
can be safely done if there is a patent crossing vein connecting
the right and left superior vena cavea. A final alternative
is to anastomose the left superior vena cava directly to the
left pulmonary artery, although the experience with this method
is limited. When unroofed coronary sinus is associated with
other major cardiac anomalies, the associated anomaly usually
presents a clear indication for operation.
RIGHT VENTRICULAR HYPERTROPHY IN CONGENITAL
HEART DISEASE AND DIFFERENTIAL
Some of the causes of right ventricular hypertrophy
include congenital heart disease (there are acquired causes
as well:see below) such as the following:
A.Triology of Fallot
Triology of Fallot is composed of:
1. Pulmonary artery stenosis (valvular)
2. Right ventricular hypertrophy (increased
thickness of the right ventricular walls)
3. Atrial septal defect (hole in the atrial
septum).
Diagnosis can be established by
Doppler echocardiography.
DD of congenital heart diseases related to
right ventricular hypertrophy
A. Cyanotic heart disease (in which the patient
has a bluish discoloration due to decreased oxygen leve from
shunting of desaturated blood from the right side of the heart
to the leftl through anabnormal hole inthe the heart or its
great vessels):
1. with right ventricular hypertrophy: Tetralogy of Fallot with
pulmonary stenosis (narrowing of the opening of the great vessel
carrying unoxygenated blood to the lungs from the right ventricle),
ventricular septal defect(hole in the lower partition separating
the right ventricle from the left, "IVS"), over-riding
of the aorta (the great vessel leading out of the heart carrying
the oxygenated blood over the interventricular septum("IVS")
to the rest of the body from the left ventricle); Eisenmenger
syndrome(see above under cyanotic heaertdisease).
B. Non-cyanotic heart disease:
1. with right ventricular hypertrophy:
ASD(hole in the atrial septum), pulmonary stenosis(narrowing
of the pulmonary artery,which receives blood from the right
ventricle ,sending to it to the lungs for oyxgenation).
Diagnosed by doppler echocardiography and MRI
Differential diagnosis
The right ventricular hypertrophy(increased
thickness of the right ventricle) can be due to congenital or
acquired causes like an atrial septal defect(congenital)and
mitral stenosis from rheumatic fever fro example.
The Abnormal Ventricular Electrocardiogram
(ECG) (see figures illustrations 1 and 2)
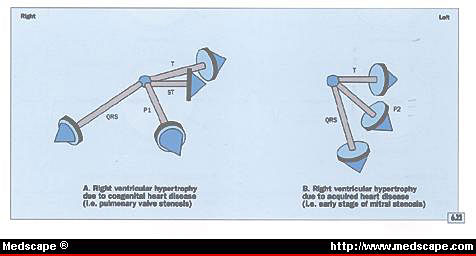
Illustration figure 6.22
The difference between the electrocardiographic abnormalities
produced by congenital heart disease, such as pulmonary valve
stenosis (A), and those produced by the early stages of acquired
disease, such as mitral stenosis (B).
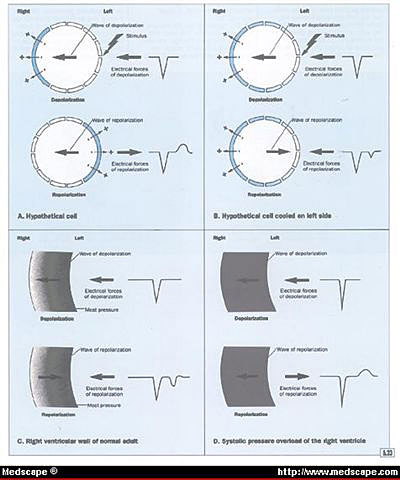
Illustration figure 6.23
Hypothetical explanation for the electrocardiographic abnormalities
caused by systolic pressure overload of the right ventricle.
The Mean T Vector and Right Ventricular
Hypertrophy
Diastolic pressure(filling period)overload
of the right ventricle should theoretically produce a mean T
vector that is larger than average, and the ST segment vector
should be parallel to the mean T vector.
Actually, the most common cause of diastolic
(filling period) overload of the right ventricle is a secundum
atrial septal defect (a hole in the upper partition of the heart,
the atrium) in which a right ventricular conduction defect dominates
the electrocardiogram. The T wave abnormality in such a patient
is secondary to the QRS abnormality, and the latter dominates
the electrocardiogram rather than abnormalities associated with
right ventricular diastolic pressure overload.
Systolic pressure (when the ventriles are
squeezig down to contract) overload of the right ventricle,
due to congenital heart disease, such as pulmonary valve stenosis,
tetralogy of Fallot, or the Eisenmenger syndrome, produces a
mean QRS vector that is directed to the right and anteriorly.
Therefore, the mean T vector will be located 150° to 180°
away from the mean QRS vector and will be directed leftward
and posteriorly (Fig. 6.22, figure 1 lnk attached).
The transmyocardial pressure gradient of the
right ventricle is decreased and finally eliminated by the abnormal
systolic pressure generated during the late stage of mechanical
ventricular systole. This permits the repolarization process
to begin in the endocardium of the right ventricle, producing
electrical forces that are opposite normal (Fig. 6.23 ,fgfure
2attached separately). A right atrial abnormality is often present.
Figure 6.22 The difference between the electrocardiographic
abnormalities produced by congenital heart disease, such as
pulmonary valve stenosis (A), and those produced by the early
stages of acquired disease, such as mitral stenosis (B).
A. The duration of the QRS complex is 0.10
second or less. The mean QRS vector is directed to the right
and anteriorly, and the ST and T vectors are directed opposite
the mean QRS vector. This type of abnormality occurs with congenital
disease, such as pulmonary valve stenosis, or advanced acquired
disease, such as mitral stenosis with moderately severe pulmonary
hypertension. A right atrial abnormality may be apparent in
patients with right ventricular hypertension.
B. The duration of the QRS complex is 0.10
second or less, and the mean QRS vector is located vertically
and posteriorly. The mean T vector may be directed to the left
and slightly posteriorly. This type of mean QRS vector is often
caused by acquired disease. A left atrial abnormality as shown
here suggests an early stage of mitral stenosis.
Figure 6.23 Hypothetical explanation for the electrocardiographic
abnormalities caused by systolic pressure overload of the right
ventricle.
A. Electrical forces and QRS and T deflections
of a hypothetical cell that has been stimulated on the left
side.
B. Electrical forces and QRS and T deflections
produced when a hypothetical cell has been cooled but also stimulated
on the left side.
C. Normal depolarization and repolarization
of the ventricular wall of a normal adult. The endocardial systolic
pressure is greatest in the endocardial area as compared to
the epicardial area. Both the QRS complex and T wave are upright.
D. Systolic pressure overload of the right
ventricle. The systolic pressure is so great that there is no
significant difference between the endocardial and epicardial
pressure. The QRS vector will be directed to the right and the
mean T vector will be directed to the left.
Early in the natural history of right ventricular hypertrophy
due to acquired heart disease, such as mitral stenosis or primary
pulmonary hypertension, the mean QRS vector tends to have an
intermediate or vertical direction; it usually retains a slightly
posterior direction. The mean T vector tends to be directed
leftward and posteriorly (Fig. 6.22 figure 1 illustratio). A
left atrial abnormality may be present with mitral stenosis,
and a right atrial abnormality may occur with pulmonary hypertension.
Later in the course of disease, as more severe
right ventricular hypertension develops, the mean QRS vector
tends to be directed more to the right and anteriorly, and the
mean T vector eventually lies 150° to 180° away from
the mean QRS vector, being directed to the left and posteriorly.
The mean ST vector tends to be parallel with the mean T vector.