These
rapid, irregular heart beats are associated with an atrial rate
of a 100 or more a minute; the ventricular rate may be less
when AV conduction is incomplete.
Supraventricular
tachycardias (SPVT) usually have narrow QRS complexes, but they
may be wide because of aberrant counduction through the intraventricular
conducting tissue, participation of a bypass tract in the intraventricular
depolarization pattern,or in the presence of a coexiting bundle
branch block.
They
originate above the bifurcation of the bundle of His. They may
be sudden and brief, persistent or chronic, lasting for seconds,
hours, to days or weeks (chronic).
The chronic and recurrent ones are related to underlying structural
causes like atrial disease or mitral disease. Persistent episodes
may be caused by drug toxicity, low potassium levels, and lung
disease.
A.
The paroxysmal SPVT tend to be recurrent and of short duration
(seconds to hours) to days or weeks:
1. SPVT due to AV nodal reentry or WPW syndrome (figure3);
2. Paroxysmal atrial flutter or fibrillation.
B.
The persistent ones (sinus tachycardia, nonparoxysmal
ectopic atrial tachycardia, multifocal atrial tachycardia, longer
episodes of PSVT or atrial flutter or fibrillation) may last
for days to weeks and may be associated with a specific contributing
pathophysiologic factor such as:
1. decompensated chronic lung disease (COPD),
2. pulmonary emboli,
3. electrolyte disturbences(low potassium levels),
4. drug toxicity (digoxin, figure 4).
C.
They
tend to be recucurrent when an underlying structural cause such
as atrial disease or mitral disease is the dominant pathophysiologic
factor.
D.
They are transient when functional abnormality
dominates (hypoxemia, heart failure, electrolyte abnormality).
E.
The chronic or long standing PSVT'S like atrial flutter or fibrillation
do not revert without treatment,often fail to revert even with
attempted treatment and if reverted will often recur despite
therapy.
F.
The most common form of paroxysmal supraventricular
tachycardia (PSVT) is AV nodal reentry due to dual pathways
of excitation in the region of the AV node (see Figure 1).
G.
There is a slow conduction pathway as well
as a fast one. When there is a disturbance in the normal conduction
through the fast pathway, the slow pathway may be activated to
conduct the excitation wave to the bundle of His, as well as retrograde
back to the fast one, and then back again down the slow pathway
continuously to produce the PSVT (see Figure 1).
| Management
of PSVT due to AV nodal reentry. |
1. In acute situations rest and sedation
plus carotid artery (one on each side of the front of the neck)
sinus massage may be adequate and effective.
2.
Medications include intravenous adenosine (see figure 2), calcium-
entry blockers, digoxin, beta-adrenergic blockers.
3.
Long term management include medications
like digoxin, propranolol (inderal) or verapamil.
Radiofrequency catheter ablation techniques are safe and effective
as well, especially for patients with poor tolerance to drugs
(see figure figure 3b radiofrequency
ablation in WPW, as well as in the treatment of atrial flutter
and fibrillation by identifying tract carrying the excitation
impulse).
Reference:Zipes,D.P.,Clinical
Application of the EKG,JACC Vol.36,No.6,2000
Supraventricular Tachycardia, Wolff-Parkinson-White
Syndrome
Synonyms and related keywords: WPW syndrome,
preexcitation syndromes, preexcitation and paroxysms of tachycardia,
accessory pathway, AP
Author: Robert Hamilton, MD, Acting Chief, Division
of Cardiology, Associate Professor, Department of Pediatrics,
The Hospital for Sick Children and University of Toronto, Canada
Coauthor(s): Shubhayan Sanatani, MD , Consulting
Staff, Division of Pediatric Cardiology, Children's and Women's
Health Center of British Columbia, Assistant Professor, Department
of Pediatrics, University of British Columbia at Vancouver
INTRODUCTION
Background:
In 1930, Wolff, Parkinson, and White
described a series of young patients who had a bundle branch
block pattern on electrocardiography (ECG), a short PR interval,
and paroxysms of tachycardia. Case reports began appearing in
the literature in the late 1930s and early 1940s, and the term
Wolff-Parkinson-White (WPW) syndrome was coined in 1940. In
1943, the existence of an accessory connection between atria
and ventricles was confirmed, which is about 50 years after
Kent's description of myocardial fibers that were believed to
conduct from atria to ventricle.
The term preexcitation was first published
by Ohnell in 1944 (the same year that the term delta wave was
coined); "preexcitation indicates an additional excitatory
spread in the ventricles of the heart, coupled to auricular
excitation." WPW syndrome is not the only form of preexcitation,
but it is the most common. Initially, through the surgical treatment
of WPW syndrome and, now in the era of radiofrequency (RF) ablation,
understanding of the pathophysiology of WPW syndrome has become
refined in the wake of these elegant descriptions.
The first surgical division of an accessory
pathway (AP) was performed at Duke University by Will C. Sealy,
MD, in 1968. The first catheter ablation was reported in 1983,
using DC energy; this was followed by the first successful RF
ablation reported in 1987.
Pathophysiology:
The underlying defect in WPW syndrome is the
presence of an AP consisting of a myocardial connection at the
atrioventricular (AV) junction. These are believed to be residual
connections from the formation of the AV junction. The primary
feature differentiating WPW syndrome from other AP-mediated
supraventricular tachycardias (SVTs) is the ability of the AP
to conduct antegradely (ie, from atrium to ventricles) and retrogradely.
The presence of this AP allows a reentrant tachycardia circuit
to be established. In orthodromic SVT, the conduction is through
the AV node to the ventricles, then back to the atria via the
AP. Because the AP can conduct in both directions, experiencing
antidromic tachycardia is also possible, in which the conduction
from atrium to ventricle occurs via the AP, resulting in a broad
complex tachycardia.
The presence of an antegrade AV connection
also allows atrial arrhythmias to be conducted to the ventricles
without passing through the AV node. Patients with WPW syndrome
can more frequently develop atrial fibrillation, which can potentially
be conducted to the ventricles rapidly (see Mortality/Morbidity).
The different patterns of preexcitation have
produced various classification systems. Classification by type
is largely obsolete, and, currently, classification by anatomic
location of the AP is used. The most common AP location is at
the left free wall.
Frequency:
Internationally:
WPW syndrome refers to preexcitation and
paroxysms of tachycardia. The WPW pattern refers to the ECG
pattern. The incidence of the WPW pattern is unknown but is
estimated to be 1-2 cases per 1000 population. This may be an
underestimate because it often represents an asymptomatic ECG
finding. The incidence of newly diagnosed cases of WPW syndrome
is approximately 4 cases per 100,000 population per year.
Mortality/Morbidity:
The incidence of sudden cardiac death
(SCD) in WPW syndrome is approximately 1 in 100 symptomatic
cases when followed for up to 15 years. Although relatively
uncommon, SCD may be the initial presentation in as many as
4.5% of cases. The cause of SCD in WPW syndrome is rapid conduction
of atrial fibrillation (AF) to the ventricles via the AP, resulting
in ventricular fibrillation (VF). AF develops in one fifth to
one third of patients with WPW syndrome; the reasons for this
and the effects of AP ablation on its development are unclear.
Certain factors increase the likelihood of
VF, including rapidly conducting APs and multiple pathways.
Cases have also been reported in association with esophageal
studies, digoxin, and verapamil. A few reports document spontaneous
VF in WPW syndrome, and SVT may degenerate into AF, thus leading
to VF; however, both scenarios are rare in pediatric patients.
The morbidity in WPW syndrome results predominantly
from AV reciprocating SVT. Even in the absence of VF, syncope
is an occasional presenting symptom. However, in most patients,
the SVT is well tolerated and not life threatening. If a patient
experiences incessant tachycardia, cardiomyopathy may develop.
Sex:
A male-to-female ratio of approximately 2:1
has been documented in some series. In other series, the syndrome
was found to be more frequent in men (1.4 per 1000) than in
women (0.9 per 1000). A recent Belgian study indicates a 3.5-fold
higher prevalence of WPW in men than in women.
Age:
People with WPW syndrome may present at any
age, with many patients presenting in infancy. A bimodal age
distribution is observed in pediatric patients, with a second
peak in school-aged children.
CLINICAL
History:
The presentations of WPW syndrome are
as diverse as an incidental finding to syncope or sudden death.
Patients usually present with symptomatic
orthodromic SVT. This is usually well tolerated and not a high
risk, especially in the pediatric population after young infancy.
The infant is often noted to be irritable,
to not tolerate feedings, or to demonstrate evidence of congestive
heart failure.
Infants often have a history of not behaving
as usual for 1 or 2 days.
An intercurrent febrile illness is often
observed.
The verbal child usually reports chest pain,
palpitations, or breathing difficulty.
Most children are previously well, and a
minority of children have a positive family history of this
condition.
Older patients can usually describe the sudden
onset of a pounding heartbeat, which is regular and "too
rapid to count." This is accompanied by a concomitant change
in their tolerance for activity.
Look for an irregular rhythm because this
may herald the presence of AF.
Physical:
During an episode of SVT, the infant
is usually tachypneic and irritable; pallor is common. The pulse
is very rapid and diminished in volume. The ventricular rate
typically is 200-250 bpm, and the blood pressure is decreased.
If the episode has been untreated for several hours, the patient
often has poor perfusion, hepatomegaly, and cardiac failure.
The child is usually anxious but hemodynamically stable. Tachypnea
often accompanies the tachycardia.
Once the arrhythmia has been terminated,
the physical examination findings are generally normal.
In several series, the incidence of associated
congenital heart disease (CHD) is reported to be as high as
30%, most commonly Ebstein anomaly of the tricuspid valve and
"corrected" transposition of the great arteries {S,L,L}.
A genetic locus linking hypertrophic cardiomyopathy
to WPW syndrome has been found on chromosome 7.
The findings of the underlying condition
often become apparent only after the SVT has been terminated,
although the hemodynamic consequences may be poorly tolerated
in the presence of CHD.
Causes:
APs are considered congenital phenomena,
which are related to a failure of insulating tissue maturation
within the AV ring. A proportion of patients with preexcitation
may have a genetic predisposition. One example of such a predisposition
is the association of preexcitation with a certain hypertrophic
cardiomyopathy locus on chromosome 7.
Preexcitation can be created surgically,
such as in certain types of Bjork modifications of the Fontan
procedure, if atrial tissue is flapped onto and sutured to ventricular
tissue.
Certain tumors of the AV ring, such as rhabdomyomas,
may also cause preexcitation.
DIFFERENTIAL
Ventricular Tachycardia
Other Problems to be Considered:
The differential diagnosis for a narrow complex
tachycardia is extensive and the term SVT is nonspecific. Automatic
versus reentrant mechanisms may be differentiated by the presence
of a warm-up or cool-down period. A regular tachycardia, allowing
for some cycle length oscillation, favors a reentrant mechanism.
The most common mechanism in pediatrics is AP-mediated SVT.
The main differential during SVT is whether the AP is concealed
(ie, conducts only from ventricle to atrium).
Few entities involve paroxysms of SVT with
a WPW ECG in sinus rhythm. Occasionally, a low atrial focus
produces the appearance of a short PR interval. The preexcitation
associated with atriofascicular APs (so-called Mahaim) is associated
with a normal PR interval. Patients with so-called Lown-Ganong-Levine
syndrome demonstrate a short PR interval but not a broad QRS
morphology. This terminology is currently out of favor but is
historically relevant.
Aberrantly conducting orthodromic SVT must
be differentiated from VT. Antidromic tachycardia must also
be differentiated from VT or Mahaim fiber tachycardia..
WORKUP
Lab Studies:
The extent of the workup is determined by
the acuity of the patient’s illness. In the patient who
has cardiogenic shock or is unconscious, direct current (DC)
cardioversion is indicated as soon as an arrhythmia is identified
to be causative. Once the patient is hemodynamically stable
or in the context of assessment following an arrest, measuring
blood gases, electrolytes, lactate levels, and drug screening
may be appropriate.
Imaging Studies:
Perform echocardiography, focusing on cardiac
function and dimensions to rule out cardiomyopathy and associated
CHD (eg, hypertrophic cardiomyopathy, Ebstein anomaly, L-transposition
of the great vessels). Significantly depressed function may
be observed in the setting of an acute arrhythmia but should
typically normalize in the absence of an incessant tachycardia.
Other Tests:
Obtaining a 12-lead ECG is necessary
in stable patients. The characteristic features are a short
PR interval, often with no isoelectric line between the end
of the P wave and the beginning of the QRS complex. The QRS
is usually broad and has accompanying ST changes. In patients
demonstrating intermittent preexcitation, ECG findings may appear
normal or even demonstrate 2 distinct QRS patterns. Several
algorithms are available to predict the location of the AP,
which assists in planning an ablation and in counseling about
the risks of the procedure. During orthodromic tachycardia,
a narrow complex QRS is evident with the P wave often detectable
as a subtle deflection within the T wave.
Further workup generally consists of Holter
monitoring to detect intermittent preexcitation and occult episodes
of SVT.
An exercise test may also be helpful in studying
the behavior of the AP at higher heart rates; however, this
test has limited predictive value.
In the presence of WPW syndrome without documented
SVT and in the presence of symptoms, a transtelephonic transient
cardiac event monitor or a longer-term monitoring system may
be appropriate.
Procedures:
An esophageal electrophysiology study can
be used to assess the behavior of the AP, the inducibility of
SVT, and the response to drug therapy. This procedure can be
performed safely as an outpatient procedure requiring only sedation.
An invasive electrophysiology study can also be performed for
these risk-stratification indications, but this is usually reserved
for patients undergoing RF ablation.
Histologic Findings:
An extremely detailed postmortem assessment
of histology from multiple sections around the AV ring may identify
APs, but this approach is impractical for assessment of every
patient with unexplained sudden death.
TREATMENT
Medical Care:
Short-term management of WPW syndrome
Patients presenting in cardiac arrest
or with hemodynamic compromise require management of the airway,
breathing, and circulation, as is standard; this includes having
a defibrillator available and providing appropriate monitoring.
Once the patient is determined to be experiencing an arrhythmia,
DC cardioversion is indicated.
In the stable patient, a variety of vagal
maneuvers may be attempted. A bag of ice slurry to the face
is very effective in infants. Older children may be able to
perform a Valsalva maneuver. Creative alternatives abound, such
as having a patient blow with his thumb in his mouth. Unilateral
carotid sinus massage may also be attempted. Ocular compression
should not be performed because it has been associated with
retinal injury.
When conservative measures fail, intravenous
access is necessary. Adenosine is the first-line agent and is
effective in approximately 90% of reentrant narrow complex tachycardias.
Adenosine must be administered as a rapid bolus because of its
short half-life. Most adenosine failure is caused by inadequate
administration of the drug. A defibrillator must be available
in the event that new arrhythmias emerge, particularly atrial
fibrillation postadenosine.
Procainamide or esmolol are available in
the resistant cases but should only be administered by physicians
familiar with these medications. Do not administer verapamil
to infants; this drug has also been reported to accelerate the
ventricular rate in AF, leading to rapid conduction that results
in VF.
Long-term management of WPW syndrome
Treatment must be individualized for
each patient and should include individual risk assessment.
Despite the importance of risk stratification to assess the
risk of sudden death, few reliable noninvasive markers exist.
The adult literature has focussed on preexcited R-R intervals
in AF as an indicator of the ability to rapidly conduct. In
a series of 60 pediatric patients, a preexcited R-R interval
of less than 220 milliseconds identified patients at high risk
for cardiac arrest; thus, if an AP can conduct impulses at a
rate of 4 per second, it can be considered a high-risk pathway.
Ambulatory monitoring and treadmill testing can provide additional
noninvasive information if the preexcitation disappears suddenly
at a discrete heart rate. However, be careful when interpreting
these noninvasive test results. Perform invasive risk assessment
in patients presenting with syncope or aborted SCD.
Long-term oral medication is the mainstay
of therapy in patients not undergoing RF ablation. Some of the
drugs available are listed below. Note that digoxin and verapamil
are contraindicated in the long-term therapy of WPW syndrome.
Surgical Care: In the era of RF ablation,
eradicating AP function in almost any patient with the WPW pattern
on ECG is feasible. However, because RF ablation is not without
its risks, perform an assessment of the risk-to-benefit ratio
for all patients. Individuals with low-risk APs, such as adults
who have never had symptoms and who do not participate in extremes
of competition, are not usually considered candidates for RF
ablation of an AP. Patient preference is the most common indication
for RF ablation in symptomatic patients not at high risk. The
procedure is relatively safe, with a complication rate of approximately
1% in most centers. Success rates range from approximately 85-95%,
with a 5% recurrence risk.
Activity:
Patients with appropriately evaluated
and treated WPW syndrome should be able to participate in all
activities, assuming that patients with high-risk pathways receive
treatment with RF ablation or a pathway-specific antiarrhythmic
agent. Generally, if a patient is significantly altering his
or her lifestyle because of the disease, he or she is probably
not receiving adequate or appropriate therapy.
In many jurisdictions, the presence of preexcitation
excludes patients from participating in the armed services and
piloting commercial flights.
MEDICATION
Emergency treatment in patients with hemodynamic
instability is directed to convert the rhythm to sinus through
a brief episode of AV block. Adenosine is the drug of choice
for immediate conversion of narrow complex SVT, but it should
not be used and is contraindicated for preexcited atrial fibrillation.
Esmolol has also been used with some success.
Beta-blockers are probably the most common
medication used to treat SVT in the presence of preexcitation.
They are moderately effective and have frequent, but rarely
life-threatening, adverse effects (except in the presence of
reactive airway disease). The efficacy of beta-blockers in reducing
the risk of accelerated conduction of atrial fibrillation in
patients with WPW syndrome is unclear. More potent medications,
such as flecainide, propafenone, sotalol, or amiodarone, may
have more effect on AP conduction or refractoriness than beta-blockers,
and they are preferred by some. The use of digoxin or verapamil
for long-term therapy appears to be contraindicated for many
WPW patients because these medications may shorten the refractory
period of an AP.
Drug Category:
Antiarrhythmic agents
-- These agents alter the electrophysiologic mechanisms responsible
for arrhythmia.
|
Category 1 Adenosine (Adenocard)
|
| Drug
Name |
Adenosine (Adenocard)
-- Slows conduction time through the AV node. Can interrupt
reentry pathways through AV node and restore normal sinus
rhythm in paroxysmal supraventricular tachycardia (PSVT). |
| Adult
Dose |
6 mg rapid IV bolus over 1-2 s initial; if no response
within 1-2 min, administer 12 mg rapid IV bolus; repeat
once with 12 mg/dose IV prn
|
| Pediatric
Dose |
Infants and children:
0.1 mg/kg IV; repeat with 0.2 mg/kg IV if first dose not
effective; not to exceed 12 mg/dose; alternatively, 0.05
mg/kg IV; if not effective within 2 min, increase by 0.05-mg/kg
increments q2min; not to exceed 0.25 mg/kg or 12 mg/dose |
| Contraindications |
Documented hypersensitivity;
heart transplant patients (known to be hypersensitive to
adenosine); second- or third-degree AV block; bradycardia;
sick sinus syndrome (except in patients with functioning
artificial pacemaker) |
| Interactions |
Coadministration with
carbamazepine may produce higher degrees of heart block;
dipyridamole may potentiate effects; methylxanthines (eg,
theophylline) may antagonize effects |
| Pregnancy |
C -Safety for use during
pregnancy has not been established. |
"untitled-1"
Precautions
Proper administration technique (ie, thoroughly
flushing IV line after rapid infusion) is essential to obtain
adequate results; adenosine-induced bronchoconstriction in patients
with asthma may occur; heart block, including transient asystole
may occur, proarrhythmia such as atrial or ventricular fibrillation
may rarely occur; cardioversion must be available during adenosine
administration
Drug Name
Esmolol (Brevibloc) -- Excellent for patients
at risk of complications from beta-blockade, particularly those
with reactive airway disease, mild-to-moderate LV dysfunction,
and/or peripheral vascular disease. Short half-life of 8 min
allows for titration to desired effect and quick discontinuation
if needed.
| Category
2 Esmolol (Brevibloc) |
| Adult
Dose |
250-500 mcg/kg/min IV
loading dose for 1 min; followed by a 4-min maintenance
infusion of 50 mcg/kg/min IV; if adequate therapeutic effect
(decreased HR and BP) not observed within 5 min, repeat
loading dose and follow with maintenance infusion using
increments of 100 mcg/kg/min (for 4 min); sequence may be
repeated q5-10min, increasing maintenance infusion by 50
mcg/kg/min with each sequence; not to exceed 200 mcg/kg/min |
| Pediatric
Dose |
Infants and children:
Limited information is available; suggested dose is 100-500
mcg/kg IV administered over 1 min initial; followed by 200
mcg/kg/min IV; titrate upward by 50-100 mcg/kg/min q5-10min
until HR or BP decreases by >10%; usual dose is 550 mcg/kg/min
(range is 300-1000 mcg/kg/min) |
| Contraindications
|
Documented hypersensitivity;
uncompensated congestive heart failure; bradycardia; cardiogenic
shock; AV conduction abnormalities; significant reactive
airways disease |
| Interactions |
Aluminum salts, barbiturates,
NSAIDs, penicillins, calcium salts, cholestyramine, and
rifampin may decrease bioavailability and plasma levels,
possibly resulting in decreased pharmacologic effect; cardiotoxicity
may increase when administered concurrently with sparfloxacin,
astemizole, calcium channel blockers, quinidine, flecainide,
and contraceptives; toxicity increases when administered
concurrently with digoxin, flecainide, acetaminophen, clonidine,
epinephrine, nifedipine, prazosin, haloperidol, phenothiazines,
and catecholamine-depleting agents
Pregnancy C - Safety for use during pregnancy has not been
established. |
| Precautions |
Beta-adrenergic blockers
may mask signs and symptoms of acute hypoglycemia and clinical
signs of hyperthyroidism; symptoms of hyperthyroidism, including
thyroid storm, may worsen when medication is abruptly withdrawn
(withdraw drug slowly and monitor patient closely) |
| |
|
Drug Name
Propranolol (Inderal) -- Class II antiarrhythmic
nonselective beta-adrenergic receptor blocker with membrane-stabilizing
activity that decreases automaticity of contractions.
| Category
3 Propranolol (Inderal) |
| Adult
Dose |
1-3 mg IV (under careful
monitoring); not to exceed 1 mg/min to avoid lowering of
blood pressure and causing cardiac standstill
Allow time for drug to reach site of action (particularly
if slow circulation); administer second dose after 2 min
prn; thereafter, do not administered additional drug in
<4 h
Do not continue doses after desired alteration in rate or
rhythm achieved; switch to PO as soon as possible; 10-30
mg PO tid/qid (usual); alternatively, administer total daily
dose as SR product qd |
| Pediatric
Dose |
0.5-1 mg/kg/d PO divided
q6-8h initial; titrate upward q3-5d prn; typical dose is
2.5-5 mg/kg/d; not to exceed 16 mg/kg/d or 60 mg/d; in older
children, total daily PO dose may be administered as SR
product qd
0.01-0.1 mg/kg IV administered over 10 min; not to exceed
1 mg (infants) and 3 mg (children) |
| Contraindications |
Documented hypersensitivity;
uncompensated congestive heart failure; bradycardia; cardiogenic
shock; AV conduction abnormalities |
| Interactions |
Coadministration with
aluminum salts, barbiturates, NSAIDs, penicillins, calcium
salts, cholestyramine, and rifampin may decrease effects;
calcium channel blockers, cimetidine, loop diuretics, and
MAOIs may increase toxicity; toxicity of hydralazine, haloperidol,
benzodiazepines, and phenothiazines may increase |
| Pregnancy |
C - Safety for use during pregnancy has not been established.
|
| Precautions
|
Beta-adrenergic blockade
may decrease signs of acute hypoglycemia and hyperthyroidism;
abrupt withdrawal may exacerbate symptoms of hyperthyroidism,
including thyroid storm (withdraw drug slowly and monitor
closely) |
Drug Name
Sotalol (Betapace) -- Class III antiarrhythmic
agent, which blocks potassium channels, prolongs action potential
duration (APD), and lengthens QT interval. Noncardiac selective
beta-adrenergic blocker.
| Category
4 Sotalol (Betapace) |
| Adult
Dose |
80 mg PO bid; gradually
increase dose q2-3d to 240-320 mg/d |
| Pediatric
Dose |
Not established; the
following doses have been suggested:
Initial: 200 mg/m2/d PO divided bid/tid; not to exceed 160
mg/d
Maintenance: 2-8 mg/kg/d (40-350 mg/m2/d) PO divided bid/tid |
Contraindications |
Documented hypersensitivity; sinus bradycardia; second-
and third-degree AV block; prolonged QT
|
| Interactions
|
Aluminum salts, barbiturates,
NSAIDs, penicillins, calcium salts, cholestyramine, and
rifampin may decrease bioavailability and plasma levels,
possibly resulting in decreased pharmacologic effect; cardiotoxicity
may increase when administered concurrently with sparfloxacin,
astemizole, calcium channel blockers, quinidine, flecainide,
and contraceptives; toxicity increases when administered
concurrently with digoxin, flecainide, acetaminophen, clonidine,
epinephrine, nifedipine, prazosin, haloperidol, phenothiazines,
and catecholamine-depleting agents |
| Pregnancy
|
B - Usually safe but
benefits must outweigh the risks. |
| Precautions
|
Beta-adrenergic blockade
may decrease signs and symptoms of acute hypoglycemia and
clinical signs of hyperthyroidism; abrupt withdrawal may
exacerbate symptoms of hyperthyroidism, including thyroid
storm (withdraw drug slowly and monitor patient closely);
caution in hypokalemia, peripheral vascular disease, hypomagnesemia,
and congestive heart failure; slower dose titration and
lower maintenance doses required in renal impairment |
Drug Name
Atenolol (Tenormin) -- Selectively blocks beta1-receptors
with little or no effect on beta2 types.
| Category 5 Atenolol (Tenormin) |
| Adult
Dose |
50 mg PO qd; increase to 100 mg/d, if necessary |
| Pediatric
Dose |
0.8-1.5 mg/kg PO qd;
not to exceed 2 mg/kg/d |
| Contraindications |
Documented hypersensitivity;
congestive heart failure; pulmonary edema; cardiogenic shock;
AV conduction abnormalities; heart block (without a pacemaker) |
| Interactions |
Coadministration with
aluminum salts, barbiturates, calcium salts, cholestyramine,
NSAIDs, penicillins, and rifampin may decrease effects;
haloperidol, hydralazine, loop diuretics, and MAOIs may
increase toxicity of atenolol |
| Pregnancy |
D - Unsafe in pregnancy |
| Precautions
|
Beta-adrenergic blockade
may reduce symptoms of acute hypoglycemia and mask signs
of hyperthyroidism; abrupt withdrawal may exacerbate symptoms
of hyperthyroidism and cause thyroid storm; monitor patients
closely and withdraw drug slowly; during an IV, carefully
monitor BP, heart rate, and ECG |
Drug Name
Amiodarone (Cordarone) -- May inhibit AV conduction
and sinus node function. Prolongs action potential and refractory
period in myocardium and inhibits adrenergic stimulation.
| Category
6 Amiodarone (Cordarone) |
| Adult
Dose Loading dose: |
800-1600 mg/d PO in 1-2
doses for 1-3 wk; then decrease to 600-800 mg/d in 1-2 doses
for 1 mo; alternatively, 150 mg (10 mL) IV over first 10
min; followed by 360 mg (200 mL) IV over next 6 h; then
540 mg IV over next 18 h
Maintenance dose: 400 mg/d PO |
| Pediatric
Dose Loading dose: |
10-15 mg/kg/d PO or 600-800
mg/1.73 m2/d PO for 4-14 d or until adequate control of
arrhythmia is attained; reduce to 5 mg/kg/d or 200-400 mg/1.73
m2/d for several wk
Limited data available for IV loading dose
Maintenance dose: 2.5 mg/kg/d PO or lowest effective dose
following loading |
| Contraindications |
Documented hypersensitivity;
complete AV block; intraventricular conduction defects |
| Interactions |
Increases effect and
blood levels of theophylline, quinidine, procainamide, phenytoin,
methotrexate, flecainide, digoxin, cyclosporine, beta-blockers,
and anticoagulants; cardiotoxicity is increased by ritonavir,
sparfloxacin, and disopyramide; coadministration with calcium
channel blockers may cause an additive effect and decrease
myocardial contractility further; cimetidine may increase
levels |
| Pregnancy |
C - Safety for use during
pregnancy has not been established. |
| Precautions |
Caution in breastfeeding
women, thyroid disease, or liver disease; may cause proarrhythmic
effect, optic neuritis, CNS toxicity, hypothyroidism, hepatotoxicity,
interstitial pneumonitis, or pulmonary fibrosis
|
Drug Name
Flecainide (Tambocor) -- Treats life-threatening
ventricular arrhythmias. Causes a prolongation of refractory
periods and decreases action potential without affecting duration.
Blocks sodium channels, producing a dose-related decrease of
intracardiac conduction in all parts of the heart with greatest
effect on the His-Purkinje system (H-V conduction). Effects
upon AV nodal conduction time and intraatrial conduction times,
although present, are less pronounced than on ventricular conduction
velocity.
| Category 7 Flecainide (Tambocor) |
| Adult
Dose |
100 mg PO q12h; may increase
by 100 mg/d q4d until adequate response achieved; not to
exceed 400 mg/d |
| Pediatric
Dose |
Initial dose: 1-3 mg/kg/d
PO or 50-100 mg/m2/d PO divided tid; may increase gradually
by 50 mg/m2/d q5d until adequate response achieved; not
to exceed 8 mg/kg/d (200 mg/m2/d)
<6 months: Initiate at lowest dose
Maintenance dose: Usually 3-6 mg/kg/d PO or 100-150 mg/m2/d
PO divided tid |
| Contraindications |
Documented hypersensitivity;
third-degree AV block; right bundle branch block when associated
with left hemiblock (bifascicular block) unless a pacemaker
is present; cardiogenic shock |
| Interactions
|
Beta-adrenergic blockers,
verapamil, and disopyramide may have additive inotropic
effects when administered with flecainide; may increase
digoxin serum levels; CYP2D6 inhibitors (eg, ritonavir,
amiodarone, cimetidine) may increase serum levels and cardiotoxicity |
| Pregnancy |
C - Safety for use during
pregnancy has not been established. |
| Precautions |
Because of proarrhythmic
effect and associated deaths, should only be used for life-threatening
arrhythmias, caution in renal or hepatic impairment (adjust
dose), CHF, and post-MI |
Drug Name
Verapamil (Calan) -- Interrupts reentry at AV
node. Restores normal sinus rhythm in patients with PSVT. Used
for short-term treatment only in children >2 y. Not intended
for long-term treatment because of shortened refractory period.
Do not use in children <2 y because of severe hypotension.
| Category
8 Verapamil (Calan) |
| Adult
Dose |
240-480 mg/d ER PO qd
or IR divided q6-8h
Alternatively, 5-10 mg IV followed by a second dose 15-30
min later if patient does not satisfactorily respond to
initial dose |
| Pediatric
Dose |
<2 years or <15
kilograms: Contraindicated
>2 years or >15 kilograms: 1-3 mg/kg PO q8h or for
rapid treatment, 0.1-0.3 mg/kg IV administered over 2 min;
may repeat q30min prn if hemodynamically stable; not to
exceed 10 mg/dose |
| Contraindications |
Documented hypersensitivity;
severe CHF; sick sinus syndrome; second- or third-degree
AV block; hypotension (<90 mm Hg systolic); IV administration
in children <2 y (deaths reported) |
| Interactions |
May increase carbamazepine,
digoxin, and cyclosporine levels; coadministration with
amiodarone can cause bradycardia and a decrease in cardiac
output; when administered concurrently with beta-blockers
may increase cardiac depression; cimetidine may increase
verapamil levels; verapamil may increase theophylline levels |
| Pregnancy |
C - Safety for use during
pregnancy has not been established. |
Precautions: Hepatocellular injury may occur;
transient elevations of transaminases with and without concomitant
elevations in alkaline phosphatase and bilirubin have occurred
(elevations have been transient and may disappear with continued
verapamil treatment), monitor liver function periodically.
Drug Name
Propafenone (Rythmol) -- Treats life-threatening
arrhythmias. Possibly works by reducing spontaneous automaticity
and prolonging the refractory period.
|
Category 9 Verapamil (Calan)
Category 10 Propafenone (Rythmol)
|
| Adult
Dose |
150 mg PO q8h initial;
may increase at q3-4d; not to exceed 300 mg q8h |
| Pediatric
Dose |
Infants and children:
Not established; the following doses have been suggested:
150-400 mg/m2/d PO divided tid/qid; may increase by 100
mg/m2/d q2-3d to achieve adequate control; not to exceed
600 mg/m2 /d; alternatively, 8-10 mg/kg/d PO divided tid/qid;
may increase by 2 mg/kg/d to achieve adequate control; not
to exceed 20 mg/kg/d |
| Contraindications |
Documented hypersensitivity;
bronchospastic disorders; conduction disorders; bradycardia;
uncontrolled heart failure; coadministration with ritonavir
or amprenavir |
| Interactions |
Inhibits CYP2D6 and may
decrease serum levels of isoenzyme substrates (eg, rifampin,
cimetidine, quinidine, warfarin); inhibitors of CYP2D6 (eg,
beta-blockers, amiodarone, paroxetine, fluoxetine, ritonavir),
CYP1A2 (eg, cimetidine, ritonavir), or CYP3A4 (eg, amprenavir,
ritonavir, erythromycin, amiodarone, fluoxetine) may increase
blood levels |
| Pregnancy |
C - Safety for use during
pregnancy has not been established. |
| Precautions |
Only use for life-threatening
arrhythmias; caution in patients with congestive heart failure,
myocardial infarction, or hepatic dysfunction (adjust dose) |
FOLLOW-UP
Further Outpatient Care:
Follow-up care with a cardiologist
is indicated for patients with WPW syndrome.
Asymptomatic patients who have a low-risk
pathway and no SVT can be monitored expectantly.
Symptomatic individuals should undergo risk
assessment and should be offered therapy according to their
symptoms. RF ablation can be curative and carried out with a
high degree of success, a low complication rate, and a low recurrence
rate.
Transfer:
Ideally, if transfer of patients with WPW
syndrome and other causes of SVT is indicated, they undergo
conversion of their rhythm in the referring institution and
are transferred in sinus rhythm.
Deterrence/Prevention:
Screening of school-aged children or athletes
through preparticipation evaluation has been suggested but,
so far, has not been considered cost-effective.
Prognosis:
Once identified and appropriately treated,
WPW syndrome is associated with an excellent prognosis, including
the potential for permanent cure through RF catheter ablation.
MISCELLANEOUS
Medical/Legal Pitfalls:
Failure to recognize this disorder
or incorrect treatment resulting in deterioration can result
in medicolegal vulnerability.
Controversies exist regarding athletes with
WPW syndrome. Risk-stratification testing has false-negative
results, with potential for adverse outcomes despite test results
in patients deemed at low risk.
Special Concerns:
Patients interested in certain professions
(eg, professional athlete, pilot) may be excluded based on WPW
syndrome.
PREEXCITATION
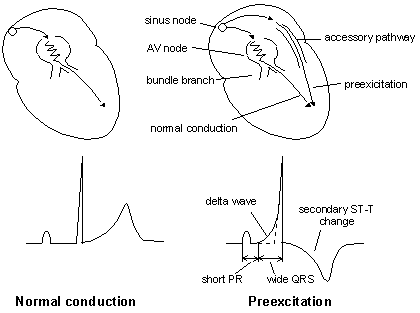
svt_fig1
The above drawing on the left depicts normal
conduction of the electrical impulse from the sinus node throuigh
the atrioventricular nodal (AV) His-Purkinje system down into
bundle branches, with the normal ECG pattern illustrated.
But the diagram on right shows the abnormal
preexcitation conduction (in the WPW syndrome) from the sinus
node through the accessory pathway (AP) with the shorter refractory
period, reaching the ventricles earlier with a short PR interval
(less than 120msec.). As a result, the onset of the QRS ECG
complex has a slurred upstroke (illustrated), causing a wide
QRS and secondary T wave inversion.
(From Jin-Ho Choi's Beginner's Guide
to Electrocardiography, Goto heartkorea.com // Index)
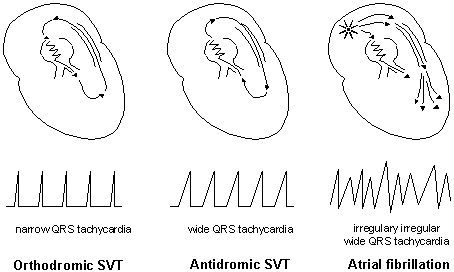
svt_fig2
The next diagram above shows how the AP can
be concealed if the electrical impulse is conducted retrograde
through the pathway and antegrade through the AV His-Purkinje
tissue to reach the ventricles, and cause a narrow QRS tachycardia
(Orthodromic supraventricular tachycardia, SVT) as shown in
the next drawings:
- svt-fig3
- svt-fig4
But if the impulse is conducted antegrade
through the AP and retrograde through the AV His-Purkinje tissue
a wide QRS complex tachycardia (Antidromic SVT) occurs (see
diagram above).
From Jin-Ho Choi's Beginner's Guide to Electrocardiography,
Goto heartkorea.com // Index.
- svt-fig5
- svt-fig6
Also, a very rapid, irregulary irregular wide
QRS tachycardia called atrial fibrillation (AF) may occur, and
can deteriorate into ventricular fibrillation VF) illustrated
above.
Steps in Location of the AP or
Bypass Tract
From Jin-Ho Choi's Beginner's Guide
to Electrocardiography, Goto heartkorea.com // Index.
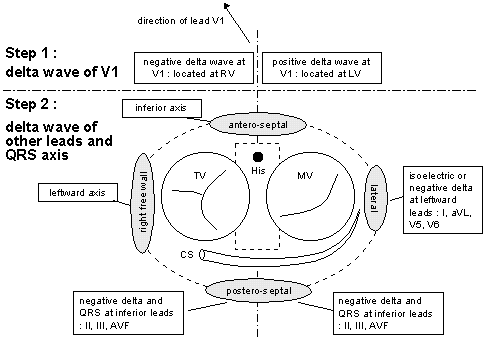
1. As shown in the illustration above, check
for the delta wave and QRS position in ECG lead V-1.
2. If the delta wave is negative at lead V-1,
the bypass tract (AP) is located in the right ventricle (RV).
See figure svt-fig4
.
a). AP is located in posteroseptal area, if
delta wave and QRS in Leads II, III, and AVF leads negative.
See figure svt-fig4
.
b). AP is in the anteroseptal area if the delta wave is in the
inferior axis.
c). If the delta wave is in the left axis,
AP is in the RV free wall.
3. If the delta wave is positive at lead V-1,
AP is in the left ventricle (LV).
a). If the delta wave and the QRS in leads
II, III, and AVF negative, the AP is in the left posteroseptal
area. See figure svt-fig3
.
b). The AP is in the lateral LV, if isoelectric
or negative delta and QRS in leftward leads I, AVL,V-5, and
V-6.
Approach to the Adult Patient with
Supraventricular Tachycardia
by Adam Zivin, MD, Best Practice of Medicine.
July 2001.
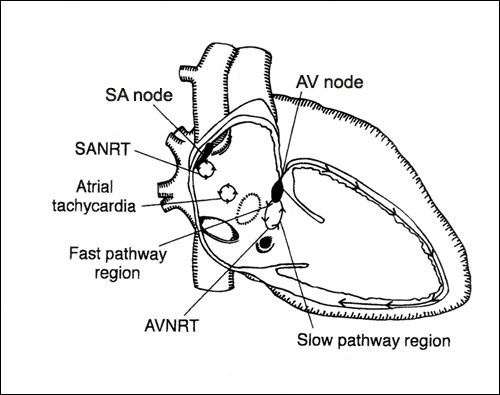 Figure
1. Possible origins of tachycardia
Figure
1. Possible origins of tachycardia
Re-entry in the sinoatrial node (SANRT), atrioventricular
node (AVNRT), and atrial myocardium (atrial tachycardia): right
anterior oblique view.
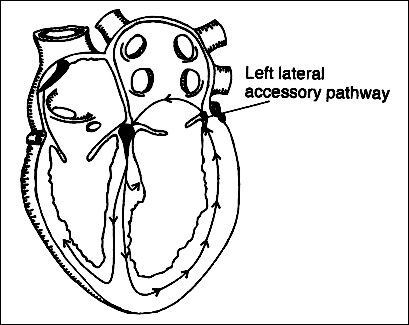
Figure 2. Possible origins of tachycardia
Orthodromic reciprocating tachycardia (ORT)
in which accessory pathway is used for retrograde conduction:
sagittal view.
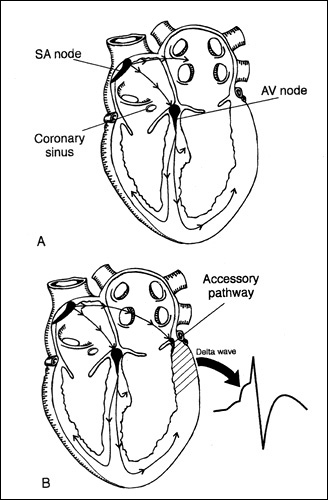
Figure 3. Normal conduction and origin
of pre-excitation
A, activation originating from the sinoatrial
node and normally conducted to the ventricles via the atrioventricular
node and the His-Purkinje system: sagittal view. B, activation
originating from the sinoatrial node and conducted to the ventricles
by both the AV node and the accessory pathway, resulting in
a fusion beat with a delta wave: sagittal view.
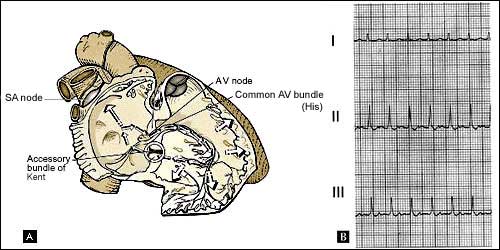
Figure 4. Orthodromic reciprocating tachycardia
using an accessory pathway
A, circuit of orthodromic reciprocating tachycardia
(ORT) causing narrow-complex tachycardia. B, limb-lead recording
from a patient with ORT owing to a concealed left-lateral accessory
pathway. Note the retrograde P wave visible ~80 msec after the
end of the QRS complex.
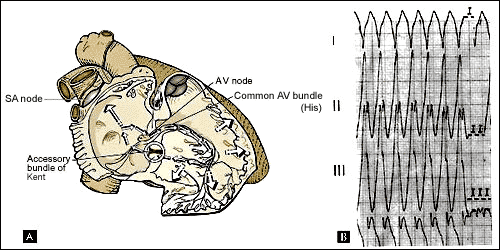
Figure 5. Antidromic reciprocating tachycardia
A, circuit of antidromic reciprocating tachycardia
(ART). B, recording of ART in a patient with a left-sided accessory
pathway.
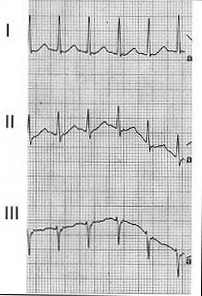
Figure 6. Limb lead recording from a patient
with AVNRT
Note the retrograde P wave buried in the terminal
portion of the QRS wave.
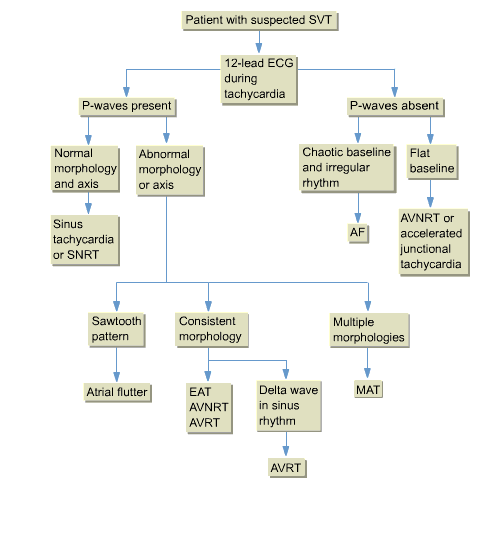
Figure 7. SVT diagnosis algorithm
AF, atrial fibrillation; AVNRT, atrioventricular
nodal re-entry tachycardia; AVRT, atrioventricular re-entry
tachycardia; EAT, ectopic atrial tachycardia; ECG, electrocardiogram;
MAT, multifocal atrial tachycardia; SNRT, sinus-node re-entrant
tachycardia.