BY
MASOOD AKKHTAR
The recording of intracavitary electrocardiographic
signals and various forms of pacing programs have experienced
enormous growth during the past 3 decades. Recordings of intracardiac
signals from the region of the His bundle, initially made by
Scherlag et al., were rapidly applied to clinical problems including
atrioventricular (AV) blocks and supraventricular and ventncular
tachyarrhythmias. Such recordings were then complemented by
pacing to unmask sinus node dysfunction and AV conduction abnormalities
as well as to initiate supraventricular tachycardias (SVTs).
Intracardiac electrophysiologic studies (EPSs) have since found
utility in a variety of cardiac arrhythmias, including sinus
node dysfunction, intraventricular and AV conduction disturbances,
SVTs, ventricular tachycardias (VTs), preexcitation syndromes,
and ventricular fibrillation (VF). Such studies are now also
employed as a prelude to correction of various arrhythmias and
conduction defects. This chapter addresses recording and pacing
techniques and their clinical utility.
TECHNIQUES OF INTRACARDIAC ELECTROPHYSIOLOGIC
STUDIES
The exact type of electric signal recordings,
specific equipment used, and pacing protocol depend upon the
nature of the clinical problem, the type of electrophysiologic
assessment, and the anticipated course of action. Routine cardiac
EPSs are performed while patients are in a nonsedated postabsorptive
state. Although some degree of sedation is advisable in apprehensive
patients, the use of drugs that may alter the properties of
the cardiac conduction system should be avoided. Antiarrhythmic
drugs are usually stopped prior to these studies. In selected
cases, antiarrhythmic drugs may be continued if a clinical event
occurred while the patient was on a specific agent. Customarily,
other cardioactive drugs that are necessary for nonarrhythmic
cardiovascular problems such as hypertension, angina, and heart
failure are continued.
The typical electrode catheters used for both recording and
cardiac stimulation are multipolar (sizes varying from 4 to
8 F). Catheters can be inserted via peripheral veins such as
the antecubital or femoral veins and, at times, the subclavian
or internal jugular veins. When a catheter is intended to be
left in place for several days, subclavian and internal jugular
veins are preferable. After using local anesthesia, a guide
wire is inserted percutaneously through a needle, and a sheath
is advanced over the guide wire. A catheter is then guided fluoroscopically
through the sheath to position in the appropriate cardiac chamber.
For most electrophysiologic testing, the catheter is placed
in the high right atrium, at the His bundle, or at the right
bundle branch region across the tricuspid valve and right ventricular
apex or outflow. For accessory pathways or AV junctional tachycardias,
a catheter is placed in the region of the coronary sinus. Heparinization
is recommended at approximately 1000 units per hour. For EPSs,
good contact between the electrodes and the walls of the various
chambers is critical. For His bundle and right bundle branch
recording, the catheter is introduced via the femoral vein,
advanced across the tricuspid valve, and gradually withdrawn
until an appropriate recording from the right bundle and/or
the His bundle is obtained (Fig.
206-1). A coronary sinus catheter can be placed via an arm,
internal jugular, or subclavian vein. If necessary, coronary
sinus catheterization can also be accomplished via a femoral
approach. Right atrial catheter placement can be done via any
of the larger peripheral veins. For a routine study, left-cases,
antiarrhythmic drugs may be continued if a clinical event occurred
while the patient was on a specific agent. Customarily, other
cardioactive drugs that are necessary for nonarrhythmic cardiovascular
problems such as hypertension, angina, and heart failure are
continued.
The typical electrode catheters used for both recording and
cardiac stimulation are multipolar (sizes varying from 4 to
8 F). Catheters can be inserted via peripheral veins such as
the antecubital or femoral veins and, at times, the subclavian
or internal jugular veins. When a catheter is intended to be
left in place for several days, subclavian and internal jugular
veins are preferable. After using local anesthesia, a guide
wire is inserted percutaneously through a needle, and a sheath
is advanced over the guide wire. A catheter is then guided fluoroscopically
through the sheath to position in the appropriate cardiac chamber.
For most electrophysiologic testing, the catheter is placed
in the high right atrium, at the His bundle, or at the right
bundle branch region across the tricuspid valve and right ventricular
apex or outflow. For accessory pathways or AV junctional tachycardias,
a catheter is placed in the region of the coronary sinus. Heparinization
is recommended at approximately 1000 units per hour. For EPSs,
good contact between the electrodes and the walls of the various
chambers is critical. For His bundle and right bundle branch
recording, the catheter is introduced via the femoral vein,
advanced across the tricuspid valve, and gradually withdrawn
until an appropriate recording from the right bundle and/or
the His bundle is obtained (Fig.
206-1). A coronary sinus catheter can be placed via an arm,
internal jugular, or subclavian vein. If necessary, coronary
sinus catheterization can also be accomplished via a femoral
approach. Right atrial catheter placement can be done via any
of the larger peripheral veins.
For a routine study, left-sided heart catheterization
is seldom necessary. In patients with VT and/or left-sided accessory
pathways, however, this is performed for diagnostic or therapeutic
purposes. Continuous heparinization is desirable for left heart
catheterization to avoid thromboembolic complications.
Electrophysiologic Recordings
Once the electrode catheters are placed appropriately, the connections
are made via a junction box and isolation units to prevent excess
current in the event of random electrical surges. All of the
electrograms are displayed simultaneously on a multi-channel
oscilloscopic recorder. In addition to the intracardiac signals,
several unfiltered surface electrocardiographic leads (i.e.,
X, Y, and Z or leads I, II, or aVF and V1) are recorded. To
reduce the noise generated with the low-frequency signals, the
usual filtering frequency for intracardiac signals is between
30 and 40 Hz for the high-pass and 500 Hz for the low-pass filters.
Although appropriately placed electrode catheters will record
desired signals at any filtering frequency, filter settings
between 30 to 40 and 500 Hz are best suited for sharp intracardiac
signals such as those from the His bundle and accessorypathways
(Fig. 206-2). Undesirable low-frequency
signals can be reduced by a high-pass filter setting of more
than 50 to 100 Hz. On the other hand, 60-cycle interference
can be eliminated with a low-pass filter setting at 50 Hz. Alteration
in the high-bandpass filter for surface electrocardiography
can markedly alter the scalar electrocardiographic morphology.
Amplification is frequently necessary to identify desirable
signals from the specialized conduction system. This can lead
to superimposition of the larger myocardial signals on various
electrocardiographic tracings. In most recording equipment,
however, limiting filters allow the adjustment of amplitude
limits.
The main value of intracardiac! electrocardiographic
tracings is timing of electric events and to determine the direction
of impulse propagation. To acquire true local electrical activity,
a bipolar electrogram with an interelectrode distance of less
than 1 cm is desirable. When unipolar electrograms are obtained,
a rapid intrinsic deflection will identify a point of local
activation. For routine intracardiac electrocardiographic studies,
unipolar electrograms provide relatively limited advantage over
bipolar signals, and therefore the latter are more often utilized.
The foregoing description relates to the routine diagnostic
invasive EPSs. In other clinical situations, different types
of diagnostic methods are employed. For example, during intraoperative
mapping, direct placement of electrodes over the epicardium
or endocardium is necessary to get appropriate signals for identifying
the precise origin and route of impulse propagation. These electrodes
can be in the form of either hand-held probes or plaques that
can be placed or sutured over the myocardium. Socks and balloons
incorporating several electrodes can also be used for epicardial
and endocardial mapping techniques, respectively. All electrical
signals can be recorded on either a disk or frequency-modulated
tape for permanent storage.
More recently, several other types of mapping
and recording equipment have emerged to locate the origin of
cardiac arrhythmias more accurately. Two of the systems likely
to find clinical utility in the mapping of arrhythmic origins
are (1) nonfluoroscopic electromagnetic endocardial mapping
(CARTO, Biosense (Cordis Webster) Marlton, NJ] and (2) noncontact
mapping (EnSite, Endocardial Solutions, Saint Paul, MN).
1. The CARTO system consists of a magnetic
field generator locator pad placed under the patient table,
a sensor-mounted catheter and a reference catheter placed intracardially,
a mapping system and a graphic computer. The catheter tip allows
orientation in relation to the reference signal. The accuracy
of catheter tip position is within a millimeter of arrhythmia
location in this low magnetic field. By moving the sensor sequentially,
one can generate a three-dimensional (3D) activation map. By
color coding, both the earliest and the latest directions of
electrical activation can be recorded. Once the initial fluoroscopy-guided
placement of reference catheter and other catheters is satisfactory,
several points are acquired. A 3D map is generated, and sensor-mounted
catheters are manipulated further without the help of fluoroscopy.
Aside from creation of an accurate map guiding the origin and
activation sequence, the CARTO system is also helpful in separating
micro from macro reentry circuits. For example, in catheter
and a reference catheter placed intracardially, a mapping system
and a graphic computer. The catheter tip allows orientation
in relation to the reference signal. The accuracy of catheter
tip position is within a millimeter of arrhythmia location in
this low magnetic field. By moving the sensor sequentially,
one can generate a three-dimensional (3D) activation map. By
color coding, both the earliest and the latest directions of
electrical activation can be recorded. Once the initial fluoroscopy-guided
placement of reference catheter and other catheters is satisfactory,
several points are acquired. A 3D map is generated, and sensor-mounted
catheters are manipulated further without the help of fluoroscopy.
Aside from creation of an accurate map guiding the origin and
activation sequence, the CARTO system is also helpful in separating
micro from macro reentry circuits. For example, in atrial flutter,
by virtue of its large circuit, the impulse propagation along
the entire route can be outlined. The atrial tachycardia, on
the other hand, can be distinguished by its radial spread from
an atrial focus. A typical map generated during this technique
is shown in Fig. 206-3, Plate
75.
2. Noncontact mapping using the Endocardial
Solutions EnSite 3000 system. The Endocardial Solutions EnSite
3000 is a new endocardial mapping system that takes a different
approach to such mapping (Fig. 206-4,
Plate 76). Like the CARTO system, the EnSite 3000 system also
makes use of an amplifier and computer system with custom software.
The EnSite catheter uses a balloon design with a 64-electrode
array arranged over the outside of the balloon. This balloon
is positioned in the center of the chamber and does not come
in contact with the walls of the chamber being mapped. Using
data from the 64-electrode array catheter, the computer uses
sophisticated algorithms to compute an inverse solution to determine
the activation sequence on the endocardial surface. Data from
all points in the chamber are acquired simultaneously.
To create a map, the balloon catheter is positioned
in the chamber and deployed. A conventional (roving) deflectable
catheter is also positioned in the chamber and used to collect
geometry information. A 5-kHz signal is emitted from the tip
electrode of the conventional catheter, and the computer analyzes
this signal to determine the position of the roving catheter
relative to the position of the balloon. The roving catheter
is moved throughout the chamber, and the location information
is collected by the system. Using this information, the computer
creates a model, called a convex hull, of the chamber during
dastole. After the chamber geometry is determined, mapping can
begin.the arrhythmia is induced, and data are acquired. The
data acquisition Process is performed automatically by the system,
and all data for the entire chamber are acquired simultaneously.
The inverse-solution computations are performed by the system
in real time and projected on to the surface of the convex-hull
model, creating a 3D model showing the activation sequence within
the chamber. Following this, the segment must be analyzed by
the operator to find the early activation or vulnerable region
of the reentry circuit. The locator technology that was used
to collect the geometry information for the convex hull can
then be used to guide an ablation catheter to the proper location
in the heart.
Because data from the entire chamber are collected simultaneously
with the EnSite 3000 system, it can be used to map nonsustained
rhythms such as premature atrial complexes, irregular rhythms
such as atnal fibrillation or polymorphic VT, and rhythms that
are not hemodynamically stable. The system is highly useful
for identifying focal arrhythmias (Fig.
206-4) and atrial flutter. Currently approved indications,
however, are for the right atrium only. The other significant
limitation of the system results from its reliance on the large-diameter
balloon catheter with its current 9.5-F lumen.
These mapping systems, both of which are relatively new, provide
electrophysiologists with new tools for diagnosing and treating
what are often complex arrhythmias. They make use of state-ofthe-art
technology to accomplish their objectives and improve the state
of the art in arrhythmia management. Because these technologies
are so new, further enhancements can be expected that will further
the usefulness of advanced mapping techniques in the practice
of electrophysiology.
Programmed Electrical Stimulation
After satisfactory placement of the electrode
catheters, patches, or other forms of recording equipment, baseline
recordings are made and programmed stimulation is initiated.
The usual site of pacing is the right atrium or left atrium
via the coronary sinus. For ventricular stimulation, the pacing
sites are the right ventricular apex, outflow tract, and rarely
some other right ventricular site. A variety of pacing programs
can be utilized, depending upon the nature of the underlying
arrhythmic problem under investigation. At least two formats
of pacing protocol are common. The first is incremental pacing,
which is pacing at a constant cycle length with gradual shortening
until the occurence of a desireable event, such as induction
of a tachycardia or production of AV block. Otherwise the incremental
atrial pacing is continued until the onset of AV nodal Wenckebach's
phenomenon: a physiologic response at faster pacing rates. Fixed-cycle-length
ventricular pacing is also used for the induction of supraventricular
tachyarrhythmias and study of ventriculoatrial conduction. Bursts
of pacing at a constant cycle length are occasionally used to
induce SVT, VT, or VF or for study of sinus node function and
integrity of subsidiary pacemakers.
The second pacing format is premature (or extra) stimulation
from atrial or ventricular sites. For the study of a physiologic
phenomenon, refractory periods, and conduction characteristics,
a single extra stimulus is usually applied after a series of
beats with a constant cycle length (Fig.
206-5). The scanning is initiated late during electrical
diastole, and the coupling interval is progressively decreased
until the atrial and/or ventricular muscle is refractory.
For induction of SVTs, single, two, or more extra stimuli are
delivered (Fig.
206-6). For the induction of VT, up to three ventricular
extra stimuli are employed. The sensitivity of pacing protocols
seems to be directly related to the number of extra stimuli
utilized.This occurs, however, at the expense of specificity
when polymorphic VT/VF can be induced at very short coupling
intervals by using multiple extra stimuli. Regardless of the
pacing protocol, the induction of sustained monomorphic VT constitutes
a specific response and is seldom induced in patients not prone
to such arrhythmias clinically. In contrast, the induction of
polymorphic VT/VF with three extra stimuli at short coupling
intervals can be nonspecific and does not provide a reliable
guide for serial testing. Both polymorphic VT and VF can be
avoided to a great extent at short coupling intervals (<200
ms) and the induction of latency between the stimulus artifact
and the local ventricular electrograms is avoided.
During routine EPSs, a variety of electrophysiologic parameters
are measured, including sinus node function and intraatrial,
AV nodal, and His-Purkinje system conduction. Initiation of
SVT and VT is attempted to determine the mechanisms, the site
of origin (by pacing and mapping techniques), and the potential
of overdrive termination as a therapy option. After baseline
studies, intravenous drugs are frequently administered to facilitate
either induction of tachycardias, aggravation of sinus node
function, or production of AV block (Fig.
206-7), or to determine drug efficacy. At the completion
of testing, the catheters are withdrawn, and gentle pressure
is applied at the area of catheter insertion. Unless arterial
catheterization is performed, patients are usually allowed to
ambulate after 4 to 6h.The role of EPSs in patient management
has evolved over the past decades from a purely diagnostic method
to a frequently applied therapeutic tool. A brief outline of
the value of clinical EPSs in various arrhythmia settings is
outlined separately under diagnostic and therapeutic categories.
INVASIVE ELECTROPHYSIOLOGIC STUDIES FOR DIAGNOSIS
Sinus Node Dysfunction
EPSs are generally performed to detect suspected
sinus node dysfunction in patients with dizziness, presyncope,
syncope, etc., in whom the diagnosis cannot be made noninvasively.
The most frequently performed test is that of sinus node suppression
by using overdrive atnal pacing. After pacing at several basic
cycle lengths for a period of approximately 30 s or longer,
the pacing is interrupted. The resultant escape interval, which
is called sinus node recovery time, is measured. By deducting
the predominant sinus cycle length from this interval, one can
obtain the so-called corrected sinus node recovery time. In
one study, sinus node recovery time in patients with sinus node
disease averaged 3087 ms, and averaged 1073 ms in normal individuals.
In another series, the value for corrected sinus node recovery
time was less than 525 ms in normal individuals and exceeded
those values in patients with overt sinus node dysfunction.
Direct sinus node recordings have been obtained by amplification
of recording from catheters placed in close proximity to the
sinus node, where both the sinus node automaticity and sinoatrial
conduction can be determined more accurately.
In the vast majority of patients with true sinus node disease,sinoatrial
conduction abnormalities are the predominant reason for sinus
node dysfunction.The sinoatrial conduction time in the absence
of obvious sinus node disease is less than 100 ms. The sensitivity
of sinus node recovery time for the detection of sinus node
dysfunction is 54 percent, whereas that of sinoatrial conduction
time is 51 percent, with a combined sensitivity of the two tests
of around 64 percent. Poor sensitivity of such testing relates
in part to the fact that, in previous studies, documented episodes
of sinus bradycardia or sinus arrest due to neurocardiogenic
mechanisms may have been included as examples of sinus node
dysfunction. The specificity of the two tests combined is approximately
88 percent. It is important to test the AV conduction in patients
with sinus node dysfunction, since the former is also frequently
abnormal. In patients with bradycardia/ tachycardia syndrome,
tachycardias are frequent, particularly those arising in the
atrium, and testing may also be necessary for the proper diagnosis
and therapy of the concomitant tachyarrhythmia.
Atrioventricutar Block
In asymptomatic patients with first-degree
AV block (prolonged PR interval), electrophysiologic assessment
is unnecessary, regardless of the QRS morphology of the conducted
beats. In asymptomatic individuals with second-degree AV block,
electrophysiologic assessment is used to find the site of the
block (Fig. 206-8 below).
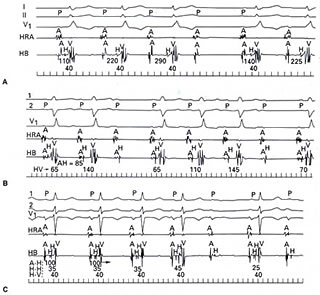
Figure 206-8
click to enlarge
Patients with intra-Hisian or infra-Hisian
block tend to have a more unpredictable course, and permanent
pacing is desirab1e. On the other hand, asymptomatic patients
with AV nodal block generally do not require permanent pacing.
Even though the intranodal block usually presents as Wenckebach's
phenomenon or Mobitz type I, it is not uncommon to see Wenckebach
phenomena within the HisPurkinje system or within the His bundle.
There is no difference in prognosis regardless of how the infra-
or intra-Hisian second-degree block manifests itself, i.e.,
type I versus type II (Fig. 206-8 above). On occasion, intranodal
blocks are preceded by no discernible change in PR interval
and from a surface electrocardiogram may appear as forms of
Mobitz type II. The absolute length of the PR interval is usually
quite diagnostic in that it is markedly prolonged (i.e., >300
ms), and there is a PR shortening exceeding 100 ms following
the block beat (Fig. 206-8 ). In symptomatic patients with second-degree
AV block, the role of EPS is limited because permanent pacing
is the appropriate intervention. On the other hand, if the patient's
symptoms cannot be explained on the basis of AV block and may
be related to another arrhythmia, such as VT, EPSs should be
considered. In patients with third-degree or complete AV block,
EPSs are seldom required, and permanent pacing is the obvious
option in symptomatic patients.
For EPSs to determine the site of AV block, it is critical to
have the catheter across the AV junction that records the His
bundle. A discernible His bundle recording enables one to determine
the exact site of AV conduction abnormality, i.e., proximal
to, within, or distal to the His bundle region. This, in combination
with surface electrocardiographic morphology of conducted beats,
enables one to identify precisely the location of conduction
abnormality. The normal atrial to His bundle activation time
(A-H) is approximately 50 to 140 ms, whereas the His to ventricular
myocardial depolarization interval (H-V) measures 35 to 55 ms.
If 1:1 AV conduction is noted during EPSs in patients suspected
of intermittent AV block, incremental atrial pacing should be
done to see whether AV block can be reproduced. AV block in
the His-Purkinje system is abnormal during incremental atrial
pacing but is a physiologic response during atrial extrastimulation
(see Fig.
206-5A) or with abrupt acceleration of atrial pacing rate.
First- and second degree blocks in the Av nodeare considered
physiologic responses during incremental atrial pacing or atriai
extrastimuiauon (see Fig.
206-5B).
Wide QRS Tachycardia
Wide QRS tachycardia occurs due to a variety
of electrophysiologic mechanisms, both from supraventricular
and ventricular mechanisms in the presence and absence of accessory
pathways (Fig. 206-9). The underlying
nature of the wide QRS tachycardia is critical for both prognosis
and therapy. EPSs have proven invaluable in distinguishing the
various etiologies (Fig. 206-10).
With few exceptions, when the nature of the arrhythmic problem
is not known and the direction of therapy is not clear, patients
with wide QRS tachycardia should undergo EPS. This is particularly
true in situations where nonpharmacologic therapy is the desired
goal.
Unexplained Syncope
Unexplained syncope is predominantly due to
cardiovascular mechanisms. The two most common reasons for cardiovascular
syncope are cardiac arrhythmias and neurocardiogenic dysfunction,
often referred to as vasodepressor syncope. Electrophysiologic
evaluation constitutes an integral part of the evaluation of
patients with unexplained syncope. During such studies, all
arrhythmic possibilities such as sinus node dysfunction, AV
conduction abnormalities, SVT, and VT should be excluded. Neurocardiogenic
mechanisms constitute the most common causes of syncope in patients
without structural heart disease, and incomplete assessment
of these patients may lead to inappropriate therapy (Fig.
206-11). The possibility of neurocardiogenic dysfunction
should always be considered in younger patients (<50 years
of age) with syncope and documented bradycardia (sinus arrest
or AV block) and can be unmasked on a tilt table. The triage
of patients toward one or the other, i.e., electrophysiologic
testing versus head-up tilt, is fairly simple and predicted
by clinical history and the presence or absence of structural
heart disease? Patients with underlying structural heart disease,
such as old myocardial infarction, primary myocardial disease,
or poor left ventricular function, generally have underlying
VT to explain the symptoms of syncope (Fig.
206-12). When arrhythmias occur in patients without overt
structural heart disease, sinus node dysfunction, AV block (particularly
intra-Hisian block), or SVTs are likely. Less frequently, VT
can occur in the absence of an overt structural heart disease.
Survivors of Sudden Cardiac Death
In most patients with documented episodes
of cardiac arrest from the onset, VF can be documented. Patients
dying suddenly generally have underlying structural heart disease
(usually coronary artery disease or primary myocardial disease)
and are prone to VT/VF due to electrical instability. It seems
prudent to investigate both the nature and extent of organic
heart disease and also to assess vulnerability to recurrent
VT/VF. At present, EPS is considered a routine part of the overall
patient assessment in this group of individuals.
EPSs in survivors of VT/VF are desirable for
a variety of reasons. Some are listed here:
1. Not infrequently, the underlying VT leading
to cardiac arrest is bundle branch reentry or BBR (Fig.
26-13). Almost 40 percent of patients with monomorphic VT
in association with idiopathic dilated cardiomyopathy and valvular
heart disease have BBR as the underlying mechanism. This arrhythmia
is preferably managed with bundle branch ablation, which is
curative, rather than with an implantable cardioverter defibrillator
(lCD) alone.
2. Several VT morphologies or other types
of tachycardia may be induced in addition to VT. Lack of awareness
of such arrhythmias may complicate patient management. For example,
the presence of rapid SVT may require separate attention to
prevent unnecessary lCD shocks.
3. In some cases, supraventricular arrhythmia
may trigger VT/VF. This may happen in patients with severe coronary
artery disease, congestive heart failure, Wolff-Parkinson-White
syndrome, etc. Elimination of the underlying causes is a more
rational therapeutic approach in such cases.
4. Patients with VT/VF often have underlying
sick sinus syndrome or AV block, which can be further aggravated
with antiarrhythmic drugs and may require permanent pacing.
Assessment for this eventuality can be done during the conduct
of an EPS and may help selection of a particular device. Because
of the increasing flexibility of these devices this need for
EPS may be less relevant in the future.
INVASIVE CARDIAC ELECTROPHYSIOLOGIC
STUDIES FOR THERAPEUTIC INTERVENTION
Because of the episodic nature of most
cardiac arrhythmias, the efficacy of any therapeutic intervention
is difficult to assess unless the arrhythmia in question can
be replicated. Diagnostic EPS provides that opportunity,and
it seems logical to use the same tool to assess therapeutic
interventions.This method to assess efficacy can be applied
for both pharmacologic and non- pharmacologic therapy.
Pharmacologic Therapy
It is arguable whether the assessment of pharmacologic
intervention is essential in patients with relatively benign
cardiac arrhythmias. The clinical course can be observed to
determine whether control has been achieved. With life-threatening
tachycardias, such as VT/VF, or with severe manifestations of
cardiac arrhythmias, such as syncope or presyncope, it is desirable
to assess efficacy of pharmacologic intervention (Fig.
26-14). The technique of drug testing has been developed
whereby the elimination of inducibility of a given tachycardia
is assessed following a drug administration. Both the drug efficacy
or inefficacy can be evaluated by this method. When drug therapy
does eliminate induction of a previously inducible tachycardia,
the addition of isoproterenol will frequently demonstrate reversal
of therapeutic drug effect. This is helpful in considering additional
beta-blocker therapy. The latter can be accomplished with ease
in patients with good left ventricular function, whereas the
addition of beta blockers may pose a problem in patients with
VT and poor left ventricular function. Failure of serial drug
testing is associated with a significant recurrence rate and
a strong incidation for nonpharmacologic intervention.
Some controversy has arisen regarding the
valuee of EPS for prediction of drug efficacy in comparison
to ambulatory monitoring. However,because of the infrequency
of spontaneous VT/VF in most patients with life-threatening
ventricular arrhythmias, ambulatory monitoring is an impractical
approach. At present, serial drug studies with multiple oral
antiarrhythmic agents are seldom carried out for SVT or VT.
Nonpharmacologic Therapy
Nonpharmacologic intervention has become
an integral part of patient management in cardiac arrhythmias.
With documented cardiac arrest from VF, implantation of an automatic
lCD is fairly common, and electrophysiologic assessment before
such therapy is routine. Both preoperative and postimplant electrophysiologic
evaluation can be done through permanent leads of an lCD through
a wand and programmer. Pacing, antitachycardia function, low-energy
cardioversion, and cardiac defibrillation can all be programmed
with newer devices. When problems are encountered following
discharge of a patient with an lCD, electrophysiologic reassessment
via lCD is frequently necessary, both for reprogramming and
for the detection of any unexplained events. For assessment
of certain other electrophysiologic parameters (e.g., AV conduction
and mechanism of SVTs), however, transvenous catheterization
may be necessary.
Patients with coronary artery disease and mappable VT are also
candidates for VT surgery when it cannot be managed with lCD,
antiarrhythmic drugs and for catheter ablation. Preoperative
EPS assessment for this possibility is important. Surgery for
VT in the form of endocardial resection or cryoablation can
be performed very effectively and relatively safely in patients
with a left ventricular ejection fraction greater than 20 percent.
This curative procedure provides effective control in approximately
75 percent of the patients who have monomorphic VT that can
be appropriately mapped, and it may be considered when other
forms of therapies are ineffective.
Surgery for SVT has gone through a significant evolution. The
introduction of catheter ablative techniques has made it rare
for patients to undergo surgery for Wolff-Parkinson-White syndrome
and/or AV nodal reentrant tachycardia. Some individuals with
resistant atrial fibrillation and flutter and those who fail
catheter ablative therapy may still be considered candidates
for such a procedure, but this is now becoming exceedingly less
frequent.
CATHETER ABLATION TECHNIQUES
The realization that the origin of VT and
SVT can be effectively mapped has made the catheter ablative
technique a rational approach. The radiofrequency form of energy
delivered through a catheter has permitted controlled trauma
to cardiac tissue to abolish or modify reentrant circuits. This
is true for both SVT and VT. Unifocal atrial tachycardia, AV
nodal reentry of all varieties, and accessory pathways including
atriofascicular fibers can be cured in over 90 percent of patients
with radiofrequency catheter ablation. Among the VTs, BBR tachycardia
seen in association with dilated cardiomyopathy (both ischemic
and nonischemic) and valvular disease is an ideal substrate
for catheter ablation. Patients with monomorphic VT associated
with myocardial scarring or other substrates can also be considered
candidates, particularly when they are not suitable for VT surgery
and have failed drug therapy. Additionally, in patients with
incessant VT or frequency VT with inadequate control despite
ICD therapy, VT ablation should be considered. By using the
electromagnetic mapping, the scarred area can be mapped during
sinus rhythm and ablation of this substrate can effectively
eliminate VT. Noncontact mapping techniques outlined earlier
are likely to further help improve ablation success rate with
unifocal or possibly multifocal tachycardias.
IATROGENIC PROBLEMS ENCOUNTERED
DURING ELECTROPHYSIOLOGIC STUDIES
Mechanical irritation from catheters
during placement and even when not being manipulated can cause
a variety of arrhythmias and conduction disturbances. These
include induction of atrial, junctional, and ventricular ectopic
beats and right bundle branch block and thus AV block in the
His-Purkinje system in patients with preexisting left bundle
branch block during right ventricular catheterization. Obviously,
AV block in the His-Purkinje system can occur in patients with
preexisting right bundle branch block during left ventricular
catheterization. Ventricular stimulation can also occur from
physical movement of the ventricular catheter coincident with
atrial contraction, producing electrocardiographic patterns
of ventricular preexcitation. Recognition of all these iatrogenic
patterns is important for avoiding misinterpretation of electrophysiologic
phenomena and the significance of findings in the laboratory.
Certain types of arrhythmias must be avoided at all costs, such
as atrial and VF. Atnal fibrillation will obviously not permit
study of any other form of SVT, and VF will require prompt cardioversion,
making it difficult to continue the EPS. If atrial fibrillation
must be initiated for diagnostic purposes (i.e., to assess ventricular
response over the accessory pathway in Wolff-Parkinson-White
syndrome), it should be done at the end of the study. Patients
with a prior history of atrial fibrillation are more prone to
the occurrence of sustained atrial fibrillation in the laboratory.
Frequently, this will occur during initial placement of catheters,
and excessive manipulation of catheters in the atria should
therefore be avoided. Catheter trauma resulting in abolition
of accessory pathway conduction or reentrant pathway may make
the curative ablation difficult or impossible.
Risks and Complications
The complication rate is relatively low when only right heart
catheterization is done, with almost negligible mortality. Other
complications include deep venous thrombosis, pulmonary embolism,
infection at catheter sites, systemic infection, pneumothorax,
and perforation of a cardiac chamber or coronary sinus. Potentially
lethal arrhythmias such as rapid VT or VF are common in the
laboratory. These are not necessarily counted as complications,
however, but are often expected and anticipated. Nonetheless,
their common occurrence makes the electrophysiology laboratory
a place for only highly trained personnel equipped to handle
such problems.