MANAGEMENT OF DEEP VEIN THROMBOSIS AND PULMONARY
EMBOLISM
Deep vein thrombosis (DVT) is a common but
elusive illness that can result in suffering and death if not
recognized and treated effectively. DVT occurs in ~2 million
Americans each year. Death can occur when the venous thrombi
break off and form pulmonary emboli, which pass to and obstruct
the arteries of the lungs. DVT and pulmonary embolism (PE) most
often complicate the course of sick, hospitalized patients but
may also affect ambulatory and otherwise healthy persons. It
is estimated that each year 600 000 patients develop PE and
that 60 000 die of this complication. This number exceeds the
number of American women who die each year from breast cancer.
PE is now the most frequent cause of death associated with childbirth.
Women are a prime target for PE, being affected more often than
men.
Deep vein thrombosis is a major complication
in orthopedic surgical patients and patients with cancer and
other chronic illnesses. DVT can be a chronic disease. Patients
who survive the initial episode of DVT are prone to chronic
swelling of the leg and pain because the valves in the veins
can be damaged by the thrombotic process, leading to venous
hypertension. In some instances skin ulceration and impaired
mobility prevent patients from leading normal, active lives.
In addition, patients with DVT are prone to recurrent episodes.
In those instances in which DVT and PE develop as complications
of a surgical or medical illness, in addition to the mortality
risk, hospitalization is prolonged and healthcare costs are
increased.
Pathogenesis of Venous Thromboembolism
Venous thrombi are intravascular deposits
composed of fibrin and red cells with a variable platelet and
leukocyte component. They usually form in regions of slow or
disturbed flow in large venous sinuses and in valve cusp pockets
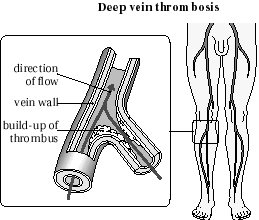
in the deep veins of the calf ( Fig 1 and Fig 1a) or in venous
segments that have been exposed to direct trauma.Venous thrombi
often break off to form PE(legveinTE-fig.1b). The formation,
growth, and dissolution of venous thrombi and PE reflect a balance
between the effects of thrombogenic stimuli and a variety of
protective mechanisms.

Valve cuspthrombus-fig1.Valve cusp thrombus
 DVT-Fig.1a
DVT-Fig.1a
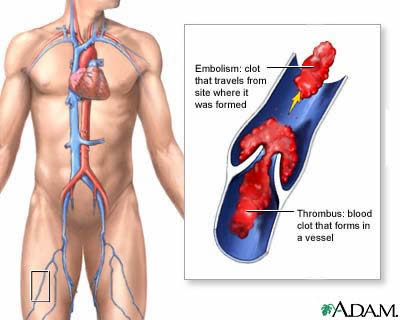
legveinTE-fig.1b:A thrombus is a blood
clot that forms in a vessel and remains there. An embolism is
a clot that travels from the site where it formed to another
location in the body. Thrombi or emboli can lodge in a blood
vessel and block the flow of blood in that location depriving
tissues of normal blood flow and oxygen. This can result in
damage, destruction (infarction), or even death of the tissues
(necrosis) in that area.
The factors traditionally implicated in the pathogenesis of
venous thrombosis are activation of blood coagulation, venous
stasis, and vascular injury. Vascular damage contributes to
the genesis of venous thrombosis through either direct trauma
or activation of endothelial cells by cytokines (interleukin-1
and tumor necrosis factor) released as a result of tissue injury
and inflammation. Blood coagulation can be activated by intravascular
stimuli released at a remote site (eg, products of injured or
infarcted tissue) or it can be activated locally by vessel wall
damage (eg, damage to the femoral vein during hip surgery) or
by cytokine-induced nondenuding endothelial stimulation. These
cytokines stimulate endothelial cells to synthesize tissue factor
and plasminogen activator inhibitor-1 and lead to a reduction
in thrombomodulin, thereby reversing the protective properties
of normal endothelium.
The thrombogenic effects of activation of blood coagulation
are amplified by stasis and counteracted by rapid flow. Venous
stasis predisposes the patient to local thrombosis by impairing
the clearance of activated coagulation factors and limiting
the accessibility of thrombin formed in veins to endothelial
protein thrombomodulin, which is present in greatest density
in the capillaries.
The mechanisms that protect against thrombosis are inactivation
of activated coagulation factors by circulating inhibitors,
dilution and clearance of activated coagulation factors by flowing
blood, inhibition of the coagulant activity of thrombin by thrombomodulin,
enhancement of the anticoagulant activity of thrombin by thrombomodulin
through activation of protein C, and dissolution of fibrin by
the fibrinolytic system.
Natural History
Venous thrombosis in the lower limb can involve the superficial
leg veins, the deep veins of the calf (calf vein thrombosis),
the more proximal veins, including popliteal veins, the superficial
femoral, common femoral, and iliac veins. Less commonly, thrombosis
involves other veins in the body. Thrombosis of the superficial
veins of the legs usually occurs in varicosities and is benign
and self-limiting. Occasionally, however, the thrombi in superficial
veins extend into the deep veins and give rise to major PE.
Deep calf vein thrombosis is a less serious disorder than proximal
vein thrombosis because thrombi in calf veins are generally
small and are therefore not usually associated with clinical
disability or major complications.
Most calf vein thrombi are asymptomatic, but these thrombi
can extend proximally and become dangerous. Venous thrombi produce
symptoms because they obstruct venous outflow, cause inflammation
of the vein wall or perivascular tissue, or embolize into the
pulmonary circulation. Extension of thrombosis is more likely
if the original thrombogenic stimulus persists.
Complete spontaneous lysis of large venous thrombi is uncommon,
and even when patients with venous thrombosis are treated with
heparin, complete lysis occurs in fewer than 10% of cases. In
contrast, complete dissolution of small, asymptomatic calf vein
thrombi occurs quite frequently.
There is a strong association between DVT and PE. Pulmonary
emboli are detected by perfusion lung scanning in ~50% of patients
with documented DVT, and asymptomatic venous thrombosis is found
in ~70% of patients with confirmed clinically symptomatic PE.
If the thrombus that embolizes is small (which is frequently
the case when it is located in the calf), the embolus is usually
asymptomatic and clinically insignificant, although the cumulative
effect, if there are repeated showers of small emboli, can cause
cor pulmonale. If the thrombus is large and involves the proximal
veins, it often produces clinical manifestations; if it is very
large or if the patient has a compromised cardiorespiratory
system, it can be fatal. Most clinically significant and virtually
all fatal emboli arise from thrombi in the proximal veins.
Venous thrombi usually organize slowly and can be complicated
by the postthrombotic syndrome. The residual abnormality can
also act as a nidus for recurrent thrombosis, which occurs in
approximately one third of patients over an 8-year follow-up
period.
Prognosis
Studies done before the introduction of anticoagulant therapy
reported that the mortality rate for PE was ~20% in hospitalized
patients with clinically obvious venous thrombosis.34 In a small
study, Kakkar and colleagues10 reported that without treatment,
~20% of silent calf vein thrombi extended into the popliteal
vein and that extension was associated with a 40% to 50% risk
of clinically detectable PE.
In a study of patients with clinically suspected DVT, Huisman
and associates reported that 6.5% (20 of 307) who had negative
impedance plethysmography at presentation developed evidence
of extension over the next 10 days. Others have reported a lower
frequency of impedance plethysmography (IPG) conversion during
serial testing. The estimated frequency of extension rate of
untreated symptomatic calf vein thrombosis is ~30%, based on
the results of these serial IPG studies.
In contrast to untreated thrombosis, the short-term prognosis
of patients with proximal DVT treated with adequate doses of
anticoagulants for 3 months is good.Clinically significant recurrent
events take place in ~5% of patients with proximal vein thrombosis
treated with an initial course of heparin followed by oral anticoagulants
or intermediate doses of subcutaneous heparin for 3 months.
Thereafter, DVT recurs in 5% to 10% of patients the year after
anticoagulant therapy is discontinued36-38 and in ~30% of patients
after 8 years.
Clinical Course in Symptomatic Patients
A comprehensive prospective follow-up study examining long-term
prognosis in consecutive patients with a first episode of documented
symptomatic DVT of the leg was recently completed by Prandoni
and associates. The study assessed the long-term incidence of
recurrent venous thromboembolism and postthrombotic syndrome.
Patients were treated with an initial course of high dose-adjusted
intravenous standard heparin or low-molecular-weight heparin
(LMWH) followed by oral anticoagulants, which were started during
the first week of treatment and continued for at least 3 months.
The dose of oral anticoagulant therapy was adjusted daily to
maintain the International Normalized Ratio (INR) between 2.0
and 3.0. All patients were instructed to wear graduated compression
stockings (40 mm Hg at the ankle) for at least 2 years. They
were seen at 3 and 6 months after presentation and every 6 months
thereafter for follow-up assessments. Patients were asked to
return immediately if they developed symptoms suggestive of
recurrent venous thromboembolism. Follow-up continued for up
to 8 years.
A total of 355 consecutive patients with a first episode of
DVT confirmed by venography were included in the study. Seventy-eight
patients experienced one or more episodes of objectively confirmed
recurrent venous thromboembolic events. Of the first recurrences,
35 (44.9%) occurred in a leg that was initially involved, 28
(35.9%) in the contralateral leg, and 15 (19.2%) were PE, which
was fatal in 9 patients (11.5%). The cumulative incidence of
recurrent VTE after 3 months was 4.9%; after 6 months it was
8.6%. The incidence of recurrent events gradually increased
to 17.5% after 2 years, 24.6% after 5 years, and 30.3% after
8 years of follow-up ( Fig 2).

Incidence of Recurrent Venous Thromboembolism-fig2 .Cumulative
incidence of recurrent venous thromboembolism after the first
episode of symptomatic deep vein thrombosis
The risk of recurrent VTE was increased by the presence of
malignancy and coagulation abnormalities and reduced in patients
who had a reversible risk factor (eg, surgery and trauma or
fracture).
Of the 355 patients, 83 developed postthrombotic syndrome and
24 developed severe postthrombotic manifestations. The cumulative
incidence of postthrombotic syndrome was 17.3% after 1 year
and 22.8% after 2 years. Thereafter, the incidence of postthrombotic
syndrome rose very gradually to 28.0% after 5 years and 29.1%
at 8 years. Thus, in more than 80% of patients manifestations
of postthrombotic syndrome became apparent in the first 2 years
after acute thrombosis. The cumulative incidence of severe postthrombotic
manifestations increased gradually from 2.6% after 1 year to
9.3% after 5 years. Thereafter, the cumulative incidence of
severe postthrombotic manifestations did not increase further.
It is likely that the use of compression stockings contributed
to this low incidence of postthrombotic syndrome, as indicated
by a recent controlled study. Ipsilateral recurrent DVT was
associated with a strong increase in risk for postthrombotic
syndrome (risk ratio 6:4).
Surprisingly, there were no significant associations between
occurrence of postthrombotic syndrome and size or location of
the thrombus. Twenty-six of the 297 patients without a malignancy
at baseline developed cancer. This occurred mainly in patients
with idiopathic DVT at presentation.
Of the 355 patients, 90 died during follow-up. The causes of
death included malignancy (n=52), ischemic stroke (n=8), acute
myocardial infarction (n=4), PE (n=9), heart failure (n=3),
anticoagulant-related hemorrhage (n=2), and miscellaneous (n=6).
In 6 patients who died suddenly, a definite cause of death was
not established.
Other studies have also reported that most recurrences take
place in patients who have idiopathic venous thrombosis or who
are exposed to a continuing risk factor (such as cancer). In
these groups, the rate of recurrence is ~15% in the 12 months
after treatment is stopped. In contrast, the long-term prognosis
in patients who develop venous thrombosis following exposure
to a predisposing cause such as surgery or trauma is very good.45
Thus, provided they are treated with anticoagulants for 3 months,
fewer than 4% of these patients develop recurrences in the following
year.
Acute Recurrent Venous Thrombosis
The label of recurrent venous thrombosis carries important prognostic
implications. Patients are usually treated with anticoagulants
for life and may suffer considerable mental anguish. Therefore,
it is important to ensure that the diagnosis of recurrent DVT
is correct. In many patients with clinically suspected recurrence,
the diagnosis of recurrence is not confirmed by objective tests.
For example, in a prospective study of patients with clinically
suspected acute DVT, almost two thirds did not have this diagnosis
confirmed by objective tests, and these patients did very well
without anticoagulant therapy.
The diagnosis of recurrent venous thrombosis can be difficult
because venography, the diagnostic standard for acute venous
thrombosis, is less reliable for diagnosis of recurrent venous
thrombosis. However, the accuracy of diagnosis of acute recurrence
has been improved by the introduction of noninvasive techniques
(see below).
Postthrombotic Syndrome
In early descriptive studies, postthrombotic syndrome was reported
to occur in ~50% of patients with symptomatic venous thrombosis.
More recently and possibly as a consequence of better initial
anticoagulation and the use of graduated compression stockings,
the incidence of postthrombotic syndrome after 8 years of follow-up
was reported to be no more than ~25%. The postthrombotic syndrome
is caused by venous hypertension, which occurs as a consequence
of recanalization of major venous thrombi leading to patent
but scarred and incompetent valves or, less frequently, persistent
outflow obstruction produced by large proximal vein thrombi.
Recanalization and valve destruction result in a malfunction
of the muscular pump mechanism, which leads to increased pressure
in the deep veins of the calf. This high pressure results in
progressive incompetence of the valves of the perforating veins
of the calf, and when this occurs, flow is directed from the
deep vein into the superficial system during muscle contraction,
leading to edema and impaired viability of subcutaneous tissues
and, in its most severe form, ulceration of venous origin. Follow-up
studies of patients with proximal vein thrombosis have demonstrated
that outflow obstruction (measured by IPG) is relieved either
by recanalization or collateral flow in 30% of patients at 3
weeks and in 70% of patients at 3 months. Valvular incompetence
is a more important cause of postthrombotic syndrome than is
outflow obstruction.
In patients with extensive thrombosis in the iliofemoral veins,
swelling may never disappear, while in patients with less severe
proximal vein thrombosis, swelling may subside after the initial
event but return in the next few years. Other manifestations
of postthrombotic syndrome are pain in the calf relieved by
rest and elevation of the leg, pigmentation and induration around
the ankle and the lower third of the leg, and, less commonly,
ulceration and venous claudication, a bursting calf pain that
occurs during exercise.
Patients with extensive thrombosis involving the iliofemoral
vein have a higher frequency of venous claudication and frequently
have greater disability than patients with more distal vein
thrombosis. However, incompetence of perforating veins may follow
thrombosis confined to calf veins and may lead to stasis changes.
In a follow-up study of calf vein thrombosis in Sweden, the
frequency of postthrombotic syndrome was reported to be 13 of
79 or 16% in 2 years' follow-up. There is evidence from recent
studies that recurrent venous thrombosis is an important risk
factor for development of postthrombotic syndrome and that risk
of developing postthrombotic syndrome is reduced by the use
of graduated compression stockings. The role of thrombolytic
therapy in prevention of postthrombotic syndrome is uncertain.
Clinical trials in acute DVT evaluating the effect of thrombolytic
therapy on subsequent development of postthrombotic syndrome
have produced equivocal results, although on balance, it is
probable that the incidence of clinical symptoms is reduced
in patients who receive thrombolysis.
The prevalence of postthrombotic syndrome in the general population
has been estimated in several countries. In Sweden it has been
reported to occur in 2% of the population, and in a study of
more than 4000 chemical-industry workers in Switzerland, the
frequency of severe venous insufficiency with venous ulceration
was reported to be between 1% and 1.5%. In an investigation
in Michigan involving more than 9000 adults older than 20 years,
the prevalence of active or healed venous ulcers was 5 per 1000.
Extrapolation of this figure to the general population in the
United States suggests that about 500 000 Americans have or
have had venous ulceration.
The diagnosis of postthrombotic syndrome is sometimes obvious
on clinical grounds if the symptoms are gradual in onset. However,
patients can have subacute symptoms of leg pain and swelling,
which may mimic acute recurrence of DVT. Although these symptoms
are usually superimposed on a background of chronic pain and
swelling, it may be difficult to exclude acute recurrence on
clinical grounds alone, and a diagnosis of postthrombotic syndrome
as the cause of the patient's symptoms can be made only after
acute recurrent venous thrombosis has been excluded.
The diagnosis of postthrombotic syndrome should include demonstration
of deep venous incompetence using Doppler ultrasound or plethysmography
and more recently by techniques such as volume plethysmography
and duplex ultrasound.
In some patients with recurrent leg pain not due to acute recurrent
venous thrombosis or postthrombotic syndrome, an alternative
cause is not found, and symptoms may be due to thromboneurosis.
This clinical syndrome tends to occur in patients who have a
morbid fear of the complications of DVT/PE. These patients may
have had a previous episode of DVT and some have evidence of
postthrombotic syndrome, but some have never had objectively
documented episodes of venous thrombosis. These patients usually
present with pain and tenderness that may be disproportionate
to physical signs of swelling. In its most severe form, patients
may be incapacitated by fear of recurrence, loss of the leg,
or death. Patients frequently have a history of multiple hospital
admissions for treatment of alleged recurrent venous thrombosis.
Many are on long-term anticoagulant therapy or antiplatelet
drugs, and some have undergone caval interruption procedures.
Unfortunately, thromboneurosis is often iatrogenic, and fear
of recurrence is reinforced each time the attending physician
admits the patient to the hospital and orders treatment based
on clinical suspicion alone. Thromboneurosis is best prevented
by ensuring that a clinical suspicion of acute venous thrombosis
(either first episode or recurrence) is always confirmed by
appropriate objective tests.
Prophylaxis
The most effective way of reducing death from PE and morbidity
from postthrombotic syndrome is to institute a comprehensive
institutional policy of primary prophylaxis in patients at risk
for VTE. Patients can be classified as being at low, moderate,
or high risk for developing VTE on the basis of well-defined
clinical criteria( Tables 1 and 2), and the choice of prophylaxis
should be tailored to the patient's risk. In the absence of
prophylaxis, the frequency of postoperative fatal PE ranges
from 0.1% to 0.8% in patients undergoing elective general surgery,
0.3% to 1.7% in patients undergoing elective hip surgery, and
4% to 7% in patients undergoing emergency hip surgery.Safe and
effective forms of prophylaxis are available for patients at
high risk, and primary prophylaxis is cost-effective.
Table 1. Risk Factors for Venous Thromboembolism
Age >60 y
Extensive surgery*
Previous venous thromboembolism
Marked immobility, preoperative or postoperative
Major orthopedic surgery
Hip surgery
Major knee surgery
Fracture of pelvis, femur, or tibia
Surgery for malignant disease
Postoperative sepsis
Major medical illness
Heart failure
Inflammatory bowel disease
Sepsis
Myocardial infarction
*Risk of postoperative thrombosis is increased by patient's
age,
presence of varicose veins, obesity, and length of surgery.
Table 2. Risk Categories for Venous Thromboembolism
| Thrombolic Event |
Category 1, Low Risk |
Category 2,
Moderate Risk* |
Category 3, High Risk |
| |
Patient younger than 40 y |
General surgery in patient older than 40 y |
Hip and major knee surgery |
| |
Uncomplicated surgery (eg, hysterectomy) |
Acute myocardial infarction |
Previous venous thrombosis |
| |
Minimal immobility |
Chronic illness
Leg fracture in a patient younger than 40 y |
Surgery for extensive malignant disease |
| Calf vein thrombosis |
~2% |
10-20% |
40-70% |
| Proximal vein thrombosis |
~0.4% |
2-4% |
10-20% |
| Fatal pulmonary embolism |
<0.02% |
0.2-0.5% |
1-5% |
*Risk increased by patient's age, length of surgery, obesity,
varicose veins, chronic illness, and postoperative sepsis.
Prophylaxis is achieved by either modulating activation of
blood coagulation or preventing venous stasis. The following
prophylactic approaches are of proven value: low-dose subcutaneous
heparin, intermittent pneumatic compression of the legs, oral
anticoagulants, adjusted doses of subcutaneous heparin, graduated
compression stockings, and LMWHs ( Table 3). Antiplatelet agents
such as aspirin are less effective for preventing VTE.
Table 3. Recommended Prophylaxis
| Low Risk |
Moderate Risk |
High Risk |
| Early ambulation |
Low-dose heparin (5000 U bid) or intermittent pneumatic
compression |
LMWH or moderate-dose warfarin or adjusted-dose heparin |
| |
|
|
*Low-molecular-weight heparin is a reasonable but more expensive
option.
?Method of choice for neurosurgery, urogenital surgery, or
if unusually high risk of hemorrhage (eg, spinal or eye surgery).
LMWH indicates low-molecular-weight heparin.
Low-dose heparin is given subcutaneously at a dose of 5000
U 2 hours before surgery and is then given postoperatively at
a dose of 5000 U every 8 or 12 hours. Low-dose heparin prophylaxis
is the method of choice for moderate-risk general surgical and
medical patients.Low-dose heparin reduces the risk of VTE by
50% to 70%; it does not require laboratory monitoring and is
simple, inexpensive, convenient, and safe. However, because
of the potential for minor bleeding, it should not be used in
patients undergoing cerebral, ocular, or spinal surgery. Low-dose
heparin is less effective than warfarin, adjusted-dose heparin,
and LMWH in patients undergoing major orthopedic surgical procedures.
Intermittent pneumatic compression of the legs enhances blood
flow in the deep veins and increases blood fibrinolytic activity.
This method of prophylaxis is free of clinically important side
effects and is particularly useful in patients with a high risk
of serious bleeding. Therefore, it is the method of choice for
preventing venous thrombosis in patients undergoing neurosurgery,
is effective in patients undergoing major knee surgery, and
is as effective as low-dose heparin in patients undergoing abdominal
surgery.
Graduated compression stockings reduce venous stasis and are
effective for preventing postoperative venous thrombosis in
general surgical patients and in medical or surgical patients
with neurological disorders, including paralysis of the lower
limbs.In surgical patients the combination of graduated compression
stockings and low-dose heparin is significantly more effective
than low-dose heparin alone. Graduated compression stockings
are relatively inexpensive and should be considered for all
high-risk surgical patients, even if other forms of prophylaxis
are used.
Moderate-dose warfarin (INR, 2.0) is effective for preventing
postoperative VTE in all risk categories. Warfarin can be started
preoperatively, at the time of operation, or in the early postoperative
period. Although the full, measurable anticoagulant effect is
not achieved until the third or fourth postoperative day, when
treatment is started at the time of surgery or in the early
postoperative period, warfarin is still effective in very high-risk
patient groups, including patients with hip fractures. Prophylaxis
with warfarin is less convenient than low-dose heparin or LMWHs
because of the need for careful laboratory monitoring.
Adjusted-dose heparin is given subcutaneously in a dose of
3500 U three times daily, starting 2 days before surgery. The
dose is then adjusted to maintain the activated partial thromboplastin
time (aPTT) at the upper limit of the normal range. Adjusted-dose
heparin is more effective than fixed low-dose heparin in patients
undergoing elective hip surgery but is less effective in preventing
proximal vein thrombosis than LMWH following elective hip surgery.
Adjusted-dose heparin is inconvenient because it requires careful
laboratory monitoring.
LMWHs have recently been approved for use as prophylactic agents
in North America. LMWHs are safe and effective for prophylaxis
in the following high-risk areas65: elective hip surgery, hip
fracture, major general surgery, major knee surgery, spinal
injury, and stroke. LMWH has been reported to be more effective
than standard low-dose heparin in general surgical patients,
patients undergoing elective hip surgery, and patients with
stroke or spinal injury. In addition, LMWHs have also been more
effective than warfarin in patients undergoing hip or major
knee surgery, and better than adjusted-dose heparin at preventing
proximal vein thrombosis after elective hip surgery.
Choice of Prophylaxis
General Surgery and Illness
Patients at moderate risk should be given prophylaxis ( Table
3 see above) with low-dose heparin. If anticoagulants are contraindicated
because of an unusually high risk of bleeding, intermittent
pneumatic compression should be used.
Hip Surgery
LMWH, oral anticoagulants, or adjusted-dose heparin is effective
following hip surgery. Of these three approaches, LMWH is the
most convenient because laboratory monitoring is not required.
Major Knee Surgery
Both LMWHs and intermittent pneumatic compression are effective
in preventing venous thrombosis in patients undergoing major
knee surgery. LMWH is more convenient and is the prophylactic
method of choice.
Genitourinary Surgery, Neurosurgery, and Ocular
Surgery
Intermittent pneumatic compression, with or without static
graduated compression stockings, is effective and does not increase
the risk of bleeding.
Diagnosis of Venous Thrombosis
A clinical suspicion of venous thrombosis should always be confirmed
by objective tests because patients with minimal leg symptoms
may have extensive venous thrombosis, whereas the classic symptoms
and signs of pain, tenderness, and swelling of the leg can be
caused by nonthrombotic disorders(legDVT-fig.1c').
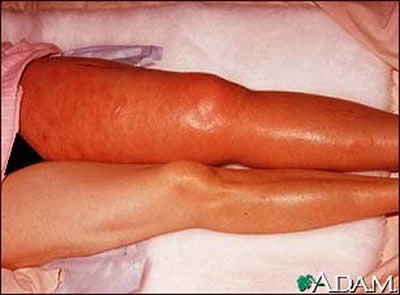
legDVT-fig.1c': This picture shows a red and swollen thigh
and leg caused by a blood clot (thrombus) in the deep veins
in the groin (ileofemoral veins) which prevents normal return
of blood from the leg to the heart.
. In most contemporary studies of ambulatory patients with
symptoms compatible with venous thrombosis, the diagnosis of
venous thrombosis is confirmed in only approximately one third
when reliable objective tests are performed. Alternative diagnoses
include superficial thrombophlebitis, cellulitis, ruptured muscle
or tendon, muscle strain, internal derangement of the knee,
ruptured popliteal cyst, cutaneous vasculitis, and lymphedema.
Despite the nonspecificity of clinical features, history and
physical examination are important components of the diagnostic
process because they may uncover an alternative cause of the
patient's symptoms and because they allow patients to be classified
as having a high, intermediate, or low probability for venous
thrombosis.With a simple clinical scoring system that included
three main components (symptoms and signs at presentation, presence
or absence of risk factors, and presence or absence of a possible
alternative diagnosis), Wells and associates showed that ~80%
of patients with high clinical probability have venous thrombosis,
while only 5% of patients with low clinical probability have
venous thrombosis. When combined with the results of noninvasive
tests, these pretest probabilities can be used to both simplify
and reduce costs of the diagnostic process ( Table 4).
Table 4. Criteria for Clinical Pretest Probabilities
| Category |
Proportion of Patients in Category (%) |
Venous Thrombosis
on Venography (%) |
High probability:
Classic clinical features and at least one risk factor |
15 |
78 |
Low probability:
Atypical clinical features and no risk factors |
60 |
5 |
Intermediate probability:
Features do not correspond to low or high probability |
25 |
23 |
Methods of Testing
Although a number of tests have been evaluated over the years,
only three have been shown to be accurate for diagnosing venous
thrombosis in symptomatic patients: venography,and venous ultrasonography.
If used properly, any one of these methods is acceptable, although
venous ultrasonography (also known as B-mode imaging) is the
diagnostic method of choice in most patients with clinically
suspected venous thrombosis.
In addition, the Simpli-red D-dimer test, which is performed
on blood obtained by finger prick at the patient's side and
which has high sensitivity and moderate specificity, shows considerable
promise as a test to rule out venous thrombosis. The D-dimer
test is often false-positive after surgery or trauma, thereby
limiting its value in these clinical situations.
The D-dimer is a specific derivative of cross-linked fibrin.A
normal enzyme-linked immunosorbent assay(ELISA) appears to be
sensitive in excluding PE.When the D-dimer level is 500 micrograms/L
or greater, the sensitivity and specficity for PE have been
shown to be 98 and 39 %, respectively.The sensitivity of the
plasma D-dimer appears to remain high up to 1 week after presentation.In
another prospective analysis,96% of 79 patients with high-probably
V/Q scans had an elevated D-dimer concentration.Thus,increased
levels of cross-linked fibrin degradation products are an indirect
but suggestive marker of intravascular thrombosis in addition
to indicating fibrinolysis.Although the sensitivity of the D-dimer
appears high,the specificity is not high enough to be diagnostic.Patients
with both suspected and provenDVT and PE often have underlying
disease states that also cause the D-dimer to be elevated.Victor
F.Tapson,MD,Pulmonary Embolism,Hurst's The Heart,10th Edition,2001,page
1628-1629.
Performance of Testing
Venography is performed by injecting radiographic material into
a superficial vein on the dorsum of the foot(venogram-fig.-1).
The contrast material mixes with the blood and flows proximally.
An x-ray image of the leg and pelvis will show the calf and
thigh veins, which drain into the external iliac vein. With
good technique, the entire deep venous system of the leg, including
the external iliac and common iliac veins, may be imaged. A
thrombus is diagnosed by the presence of an intraluminal filling
defect.
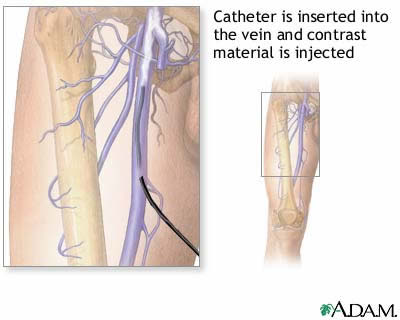
venogram-fig.-1:Leg venography is a procedure where contrast
material is injected through a catheter in a vein to help visualize
the internal structures by using x-rays. The test is used to
identify and locate thrombi (blood clots) in the veins of the
extremity that is affected.
Impedance plethysmography is performed by placing two sets
of electrodes around the patient's calf and an oversized blood
pressure cuff around the thigh. The electrodes sense a change
in blood volume (increased blood volume decreases electrical
impedance) in the calf veins, which is recorded on a strip chart.
Changes in venous filling are produced by inflating the thigh
cuff to obstruct venous return and then reestablishing blood
flow by deflating the cuff and assessing the time taken for
venous volume in the calf to return to baseline. If an occlusive
thrombus is present in the popliteal or more proximal veins,
venous emptying is delayed. The test may also detect extensive
calf vein thrombosis if venous outflow is obstructed, but it
fails to detect the majority of calf vein thrombi.
Venous ultrasound imaging of the venous system is obtained
with high-resolution equipment to produce two-dimensional images
by real-time computation of reflected signals from an array
of ultrasound sources. The ultrasound probe is first placed
over the common femoral vein in the groin(DopplerUS-fig.2).
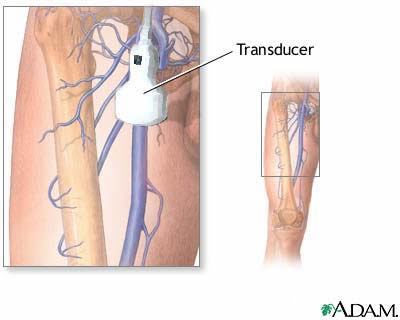
DopplerUS-fig.2:Doppler ultrasonography examines the blood
flow in the major arteries and veins in the arms and legs with
the use of ultrasound (high-frequency sound waves that echo
off the body). It may help diagnose a blood clot, venous insufficiency,
arterial occlusion (closing), abnormalities in arterial blood
flow caused by a narrowing, or trauma to the arteries.
The transducer is then moved distally to visualize the superficial
femoral vein over its course. The entire popliteal vein is then
visualized in the popliteal fossa and traced distally to its
trifurcation with the deep veins of the calf. Gentle pressure
is applied with the probe to determine whether the vein under
examination is compressible. The most accurate ultrasonic criterion
for diagnosing venous thrombosis is noncompressibility of the
venous lumen under gentle probe pressure Vein compressibility
is best evaluated in the transverse plane. Visualization of
the proximal portion of calf veins can often be achieved by
experienced operators, but resolution can be suboptimal, and
the sensitivity and specificity of venous ultrasonography is
much lower for calf vein thrombosis than for proximal vein thrombosis.
Duplex ultrasound, which combines real-time imaging with pulsed
gated Doppler and color-coded Doppler technology, facilitates
identification of veins, and as technology improves, diagnostic
accuracy for calf vein thrombosis may increase. Although it
has been claimed that color-coded Doppler is accurate for calf
vein thrombosis, this contention has not been demonstrated by
an appropriately designed clinical study.
Physiology of Doppler Study of Lower Extremity Veins
Spontaneity: When the Doppler is placed over a large vein,
a spontaneous venous flow signal should be heard. Minor repositioning
should be all that is necessary to obtain a detectable flow
signal in most veins. If more extraordinary measures are needed,
such as elevating, compressing, or another manipulation of the
limb, this suggests an abnormality in venous flow.
Phasicity: Venous return varies with the respiratory cycle.
Above the diaphragm. there is an increase in venous return during
inspiration. Below the diaphragm, venous return decreases during
inspiration because increased intraabdominal pressure during
inspiration opposes venous return. A loss of phasicity with
respiration suggests venous obstruction.
Augmentation: If a Doppler is placed over a vein in the proximal
limb (for example, the femoral vein) and a distal portion of
the limb (for example, the calf) is compressed, there should
be an increase in venous return. This phenomenon, which is called
augmentation, occurs only if the vein is patent between the
site of compression and the site of Doppler interrogation.
Competency: If a normal limb is compressed proximally (for
example, over the thigh) or if a Valsalva maneuver is performed,
the Doppler flow signal obtained distally (for example, over
the popliteal vein) should cease temporarily as flow is stopped
by the closure of venous valves. If the valves are incompetent,
a retrograde flow signal will he noted.
Pulsatility :Unlike arterial flow, venous flow is not necessarily
pulsatile. When significant pulsatility is noted, one must consider
the possibility of tricuspid regurgitation, right-sided heart
failure, pulmonary hypertension, volume overload, an arteriovenous
fistula, or other causes of increased venous pressure with pulsatility.
Venography is the reference standard, but it is invasive; the
other two tests are noninvasive. All three tests are sensitive
and specific for proximal vein thrombosis (thrombi in the popliteal
and more proximal veins) in symptomatic patients, although IPG
is less sensitive and less specific than venous ultrasound.
Venography detects calf vein thrombosis. Venous ultrasonography
detects ~50% of symptomatic calf vein thrombosis; sensitivity
is said to be higher in the hands of some experts, but this
impression awaits confirmation in large clinical trials. Impedance
plethysmography is insensitive to calf vein thrombosis, detecting
<20%. Venous ultrasonography is now the diagnostic method
of choice in patients with symptoms suggestive of DVT.
Venography can be painful, it is relatively expensive and inconvenient
to perform, and, on rare occasions, can be complicated by phlebitis.
In addition, when performed by nonexpert radiologists, up to
30% of venograms are technically inadequate and therefore impossible
to interpret. In contrast, venous ultrasonography is readily
available, painless, and can be performed at bedside. However,
like venography, this test is operator dependent.
There is evidence from diagnostic studies using serial noninvasive
testing in patients with symptoms of DVT that calf vein thrombi
are not dangerous, provided that they remain confined to calf
veins. However, calf vein thrombi can extend and do so in ~30%
of cases. Because only ~5% of patients with symptoms of DVT
have calf vein thrombosis ( Fig 3),
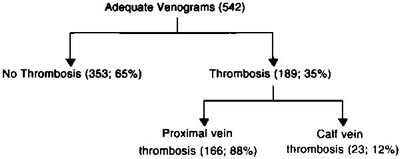
Fig 3. Location of venous thrombi in symptomatic outpatients.
Reprinted from Cogo et al
it is safe to exclude clinically important venous thrombosis
if the venous ultrasonography is negative at presentation in
patients who have low pretest clinical probability, because
the negative predictive value of a negative venous ultrasound
is more than 99%. In patients at moderate or high clinical probability,
however, it would be prudent to repeat the test once after 5
to 7 days to detect the small percentage of patients with calf
vein thrombosis that extends ( Fig 4).
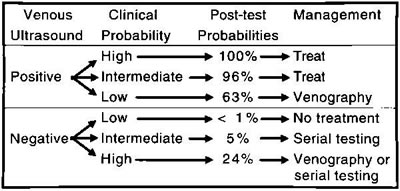
Fig 4. Diagnostic approach to deep vein thrombosis
The safety of withholding treatment when either the IPG or
venous ultrasound test result is negative at presentation and
subsequently on repeated testing over the next week has been
demonstrated in a number of well-designed studies.Between 1%
and 2% of patients with negative IPG at presentation and <1%
of patients with negative venous ultrasonography develop clinically
important events during the first 7 days of serial testing.
When these patients with negative venous ultrasonography (or
IPG) are followed up after 6 months, 99% have had no recurrences
( Fig 5).
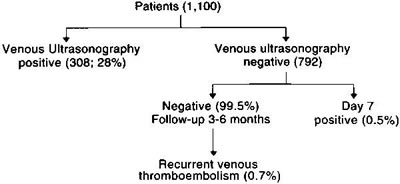
Fig 5. Management of clinically suspected deep vein thrombosis
with venous ultrasonography at presentation and on day 7.
Diagnosis of Recurrent Venous Thrombosis
The diagnosis of clinically suspected recurrent venous thrombosis
is often more difficult to establish than diagnosis of the first
episode of venous thrombosis. As with patients with suspected
acute venous thrombosis, most patients referred with a diagnosis
of recurrence do not have recurrent venous thrombosis. The clinical
diagnosis of recurrent venous thrombosis is less specific than
the diagnosis of the first episode of venous thrombosis48 because
patients fear recurrence and physicians are sensitized to the
possibility of this diagnosis. As a consequence, there is a
tendency to overdiagnose recurrent venous thrombosis by attributing
any new episodes of leg pain or swelling to a recurrent episode.
Any other cause of leg pain or swelling can be confused with
recurrence, but the most important mimic is postthrombotic syndrome,
particularly because this disorder occurs in ~30% of patients
who have experienced proximal vein thrombosis. The most common
manifestations of postthrombotic syndrome, chronic aching and
swelling of the calf, are unlikely to be confused with recurrent
venous thrombosis. However, subacute exacerbations of pain and
swelling can occur after episodes of increased activity or sometimes
without an obvious precipitating cause and can be difficult
to differentiate from recurrence. Because of their fear of recurrent
venous thrombosis, patients often become concerned if they develop
even minimal exacerbations of symptoms or signs. Finally, some
patients develop recurrent episodes of superficial phlebitis
or local cellulitis, which can be confused with recurrent DVT.
For these reasons, and because overdiagnosis of recurrent venous
thrombosis often results in unnecessary prolongation of anticoagulant
treatment, every effort should be made to confirm a diagnosis
of suspected recurrence.
The diagnosis of recurrent venous thrombosis is made or excluded
by a combination of either IPG and venography or venous ultrasonography
and venography (see Fig 6 below)

Fig 6. Diagnosis of recurrent venous thrombosis. *On venous
ultrasonography; if positive in a venous segment that had been
compressible on previous assessment.
A correct diagnosis of recurrent venous thrombosis is made
by repeating the test used to make the initial diagnosis when
the patient presents with suspected recurrence. The diagnostic
process is facilitated by obtaining a baseline noninvasive test
(either IPG or venous ultrasonography) when anticoagulants are
discontinued and repeating the test if it is still abnormal
at this time.The negative test result can then be used as a
baseline against which future tests can be compared.
The rate of conversion is different for IPG and venous ultrasonography.
The IPG result is negative in 60% of patients with proximal
vein thrombosis by 3 months and in 90% by 12 months. The rates
of conversion for venous ultrasonography are lower than those
for When the results of IPG or venous ultrasound are negative
before presentation with a suspected recurrence, a positive
result can be used to make a diagnosis of recurrent venous thrombosis.
If the IPG performed at the previous visit was abnormal and
remains abnormal at presentation with suspected recurrence,
further testing with venography is required; if there is a new
intraluminal filling defect, a diagnosis of recurrence can be
made. If the results of venous ultrasound were abnormal at the
previous visit, it is often possible to diagnose recurrence
by demonstrating extension into a previously normal venous segment
or by an increase in diameter of the venous lumen in a previously
affected segment. Recurrence can be excluded if venography shows
either no change or improvement compared with the previous examination
or if a negative IPG or venous ultrasound remains negative on
serial testing over the next 7 days (see Fig 6 above).
Diagnosis of Pulmonary Embolism
The clinical diagnosis of PE is also highly nonspecific because
the clinical features may be simulated by other cardiorespiratory
or musculoskeletal disorders.Accordingly, the diagnosis should
always be confirmed by objective tests.
Patients may present with clinical features of minor or major
PE. Patients with minor PE can have one or a combination of
the following symptoms: transient shortness of breath, sharp
localized chest pain aggravated by inspiration (pleuritic-type
pain), and hemoptysis. The clinical features of minor PE are
nonspecific and can also occur in patients with viral or bacterial
pulmonary infections, postoperative atelectasis and pneumonia,
acute bronchitis, and musculoskeletal chest wall pain. Esophageal
spasm can cause severe chest pain that is not usually aggravated
by breathing but may be confused with PE. Pleuritic-type chest
pain may accompany pericarditis or immune pleuritis. In addition,
patients with a past history of VTE may suffer anxiety attacks
that are manifested as shortness of breath and occasionally
as chest pain. These patients often have fleeting attacks of
sharp chest pain that last for seconds or a feeling that they
cannot take a deep breath.
Patients with chronic obstructive lung disease who become acutely
short of breath or develop pleuritic-type chest pain or hemoptysis
present a difficult problem, because all of these complications
can be produced by chest infection as well as by PE. Likewise,
it can be difficult to differentiate between postoperative PE
and postoperative atelectasis and infection, because both of
these disorders can cause shortness of breath and pleuritic-type
chest pain.
Patients with major PE usually have severe shortness of breath
with or without associated right-heart failure. Patients who
sustain a massive embolism or have impaired cardiorespiratory
reserve and sustain a moderate-sized embolus may present with
hypotension, syncope, and peripheral circulatory failure. Sometimes
there is associated dull central chest pain.
Some of these features also occur in patients with acute myocardial
infarction, a fulminating pneumonia, dissecting aortic aneurysm,
pericardial tamponade, a massive hidden bleed, or septic shock.
PE may also present with nonspecific manifestations such as
arrhythmia, fever, unexplained heart failure, mental confusion,
or, rarely, as bronchospasm.
Approach to Diagnosis of Pulmonary Embolism
The most reliable test for diagnosis of PE is pulmonary angiography,
because a normal well-performed pulmonary angiogram excludes
the diagnosis of PE, whereas demonstration of a constant intraluminal
filling defect in a pulmonary artery establishes diagnosis(DIAGPEANGIOG-fig.9).
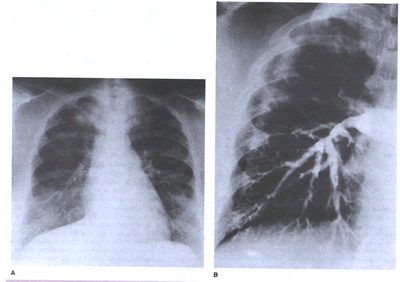
DIAGPEANGIOG-fig.9:: Pulmonary hypertension due to organized
clot in central pulmonary arteries. Dramatic relief after pulmonary
thromboendarterectomy. A. Chest radiograph. The right upper
lobe is strikingly hypoperfused, and the vasculature on the
left is quite prominent, reflecting redirection of the pulmonary
blood flow to open vessels. B. Angiogram. The flow to the right
upper lung is interrupted by the large central clot.
However, pulmonary angiography is expensive, invasive, and
not readily available in most hospitals and unavailable in many.
Therefore, other less direct approaches are usually taken. The
most useful test is the perfusion lung scan(DIAGPEPERFScan-fig10),
because if the test result is normal, diagnosis of PE is excluded.
However, before the scan is performed, the patient should have
a thorough clinical evaluation, because the combination of clinical
probability and pulmonary scanning is important in clinical
decision making. Using clinical features, presence or absence
of risk factors, and presence or absence of features that suggest
an alternative diagnosis, it is possible to classify patients
into three groups: high, low, and intermediate probability.
In addition, the patient should undergo chest radiography(DIAGPEXray-fig.8')
and electrocardiography. Although the latter tests are often
not helpful, they can be useful in ruling out other disorders
that simulate PE. In addition, a chest radiograph is required
for proper interpretation of the perfusion lung scan.
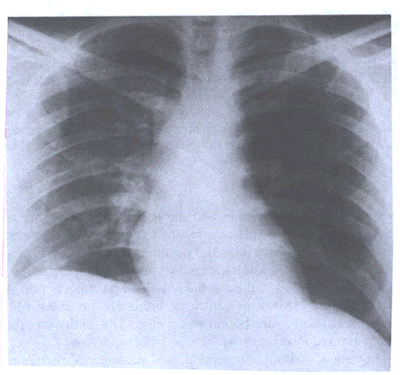
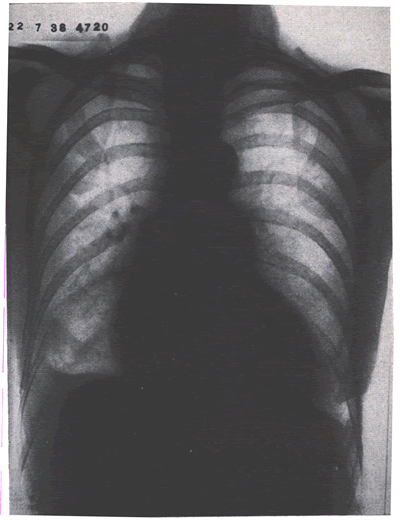
DIAGPEXray-fig.8': Patient with massive pulmonary embolism
obstructing the left main pulmonary artery.Note the uneven distribution
of pulmonary blood flow between the two lungs in favor of the
right.
A second approach, which is complementary to the first, is
to look for a source of PE in the deep veins of the leg with
either venous ultrasound or venography. This approach can be
very helpful, because although <20% of patients with proven
PE have clinical symptoms or signs suggestive of leg vein thrombosis,
~70% have venographic evidence of venous thrombosis.
The perfusion scan remains the pivotal test(DIAGPEPERFScan-fig10).
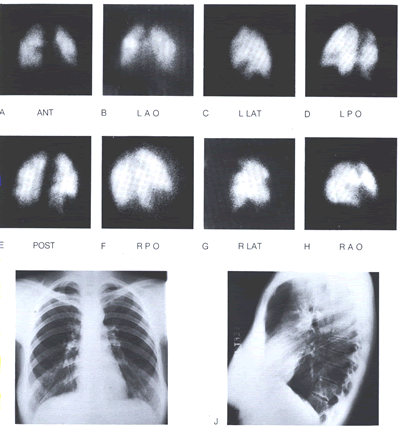
DIAGPEPERFScan-fig10:. PULMONARY THROMBOEMBOLISM. Many well
defined segmental perfusion defects involving both lungs (A
through F) with normal chest x-ray (I and J) and normal lung
ventilation study. This is a classic pattern of pulmonarv embolism.
NORMAL LUNG PERFUSION AND VENTILATION
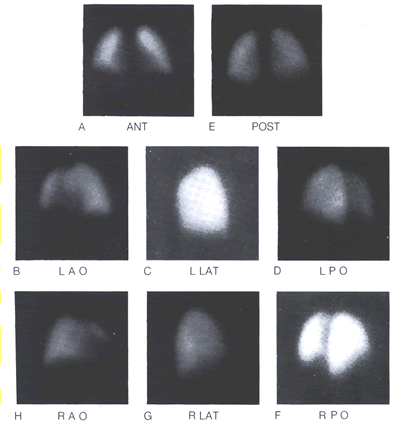
diagpeperfscan-fig11. NORMAL LUNG PERFUSION. An optimal routine
examination includes 8 views (A through H). The posterior obliques
(D and F) are the most valuable views, and the anterior obliques
(B and H) are usually the least useful. When the patient is
injected in the supine position, the radioactive particles are
evenly distributed throughout the lungs, with a gently increasing
gradient of activity from the upper anterior to the lower posterior
lung fields. The cardiac and mediastinal spaces between the
lungs have a configuration in the combined anterior and posterior
views (A and E) that is similar to the respective area in the
PA chest x-ray. Cardiomegaly and mediastinal masses will cause
distortions that are common to both examinations. An enlarged
cardiac space may be caused by cardiomegaly, and by effusions
or other conditions of the pericardial sac and adjacent pleural
cavity. When a diverging hole collimator is used, the oblique
views show the nearer lung larger than the opposite lung. In
the lateral views (C and G) the smaller image of the distant
lung is superimposed on the closer lung and normally is not
identifiable if the patient is in the true lateral position.
If rotated, the lung images may override and cause a false defect,
which will disappear when the patient is repositioned accurately.
However, a true lesion is unlikely if seen in only one view
of a complete study.
If the perfusion scan is normal( see diagpeperfscan-fig11 above),
the diagnosis of PE is excluded. If the perfusion scan is abnormal,
then the diagnostic approach depends on the clinical probabilities
and the size and V/Q pattern of the defect. A diagnosis of PE
can be made if the lung scan shows a segmental or greater perfusion
defect and normal ventilation and the clinical probability is
high or intermediate. A decision can be made to exclude a diagnosis
of PE if clinical probability is low and the perfusion defect
is small, particularly if it is matched (low-probability defect)
( Table 5).
Table 5. Probabilities of Pulmonary Embolism
Based on a Combination of Clinical Impression and Lung Scan
Findings
Clinical
Suspicion |
High |
Intermediate |
Low |
Low |
High |
Other
Combinations
|
| Lung scan |
High |
High |
Low |
High |
Intermediate*
or low |
? |
| Likelihood of PE (%) |
96 |
80-88 |
2-6 |
50 |
|
10-50 |
*Hull RD 1983,3 PIOPED 1990.
All other combinations of clinical and lung scan probabilities
require further investigation before a diagnosis of PE can be
ruled in or out. In such patients, venous ultrasonography or
venography is useful because a positive result allows a diagnosis
of VTE to be made. Unfortunately, a negative test result for
venous thrombosis cannot be used to rule out a diagnosis of
PE because tests for venous thrombosis are negative in ~30%
of patients with established PE. The venogram or venous ultrasound
may be negative for venous thrombosis in these patients because
the source thrombus has embolized completely or because it originated
in the deep femoral, internal iliac, or renal veins or the inferior
vena cava, which are not usually visualized by venography. Alternatively,
the embolism could have originated in upper limb veins, the
right side of the heart, or the pulmonary arteries.
Electrocardiography and Chest Radiography
With PE, the ECG is often normal or shows nonspecific changes.
In patients with pericarditis or acute myocardial infarction,
ECG changes may be diagnostic. In the appropriate setting, ECG
changes of acute right-heart strain strongly suggest PE(DiagPEECG-fig.8''').

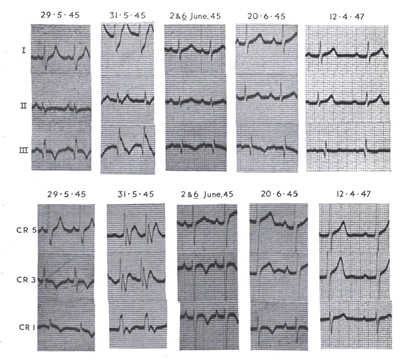
DiagPEECG-fig.8''':Electrocardiogram showing the characteristic
appearances associated with massive pulmonary embolism (lead
IV-R=CR4; LP-R=CRa-3; RP-R=CRi).
The diagnosis of acute right ventricular stress may be proved
electrocardiographically (DiagPEECG-fig.8''').Limb leads show
sinus tachycardia,a constant S wave in lead 1, a frequent Q
wave in lead 3, inversion of T3, flattening or slight inversion
of T2i and rather low voltage . Occasionally P 2 becomes tall
and sharp . These appearances are not unlike those of posterior
myocardial infarction, although an absent S1, conspicuous Q
2, and elevation of the R-T segment in lead 3 should be sufficient
to distinguish the latter in standard leads. Again, Q3 in cases
of massive pulmonary embolism is caused by cardiac rotation,
and is not seen in lead VF. In multiple chest leads appearances
are equally characteristic : the T wave is nearly always inverted
in leads V1-3 over the right ventricle, sometimes in V4 and
occasionally even in V5 (DiagPE ECG-fig.8''' above); and clockwise
rotation or displacement of the interventricular septum to the
left brings the RS pattern round as far as V5 or even V6. There
are no pathological Q waves and the RS-T segment is not deviated
from the baseline; but in about 15 per cent of cases there is
transient right bundle branch block (DiagPE ECG-fig.8'''' below).
These changes are not immediate, but develop within a few hours,
and are usually maximum within one to three days. Recovery is
relatively slow,three to six weeks elapsing before the T wave
is finally upright again in leads V1 or V2.
DiagPEECG-fig.8'''' :Electrocardiogram showing transient right
bundle branch block in a case of massive pulmonary embolism.
The chest radiograph is rarely, if ever, diagnostic(DIAGPEXray-fig.8').
It may show a pneumothorax, pulmonary edema, or findings suggestive
of primary or secondary malignancy. The finding of a Hampton
hump (a semicircular opacity with the base abutting the pleural
surface) is strongly suggestive of pulmonary infarction(DiagPEXray-fig.8''),
but in the vast majority of patients chest radiography findings
are nonspecific or normal. Other radiographic features compatible
with PE include pleural effusion, subsegmental atelectasis,
pulmonary infiltrate, raised hemidiaphragm, regions of apparent
oligemia(DIAGPEXray-fig8 ,see above), or a prominent pulmonary
vascular shadow at the hilum. However, none of these features
are diagnostic of PE because they can be produced by other conditions,
including obstructive lung disease, pulmonary infection, or
atelectasis.
DiagPEXray-fig.8'': -Skiagram showing a small pulmonary infarct
at the right base with a little hemmorrhagic effusion.
Arterial Blood Gases
Measurement of arterial blood gases in patients with PE is
rarely useful because arterial blood gas measurements lack specificity
and are only moderately sensitive for PE. Hypoxemia and hypocarbia
occur in conditions that simulate PE, and arterial oxygen tensions
can be normal in patients with minor PE.
The diagnosis of acute PE cannot be excluded based on a normal
PaO2,and although theAlveolar-arterial(A-a) difference is usually
elevated,it may very rarely be normal in patients without preexisting
cardiopulmonary disease.An important tenet should be that unexplained
hypoxemia,particularlyy in the setting of risk factors for DVT,should
suggest the possibility of PE.
Significant hypoxemia excludes hyperventilation as the cause
of the patient's symptoms, although this condition is rare.
Victor F.Tapson,Md,Pulmonary Embolism,Hurst's
The Heart,10th Edition.2001,Pages1629.
Lung Scans
Perfusion scanning is performed by injecting isotopically labeled
human macroaggregates of albumin intravenously. The macroaggregates
are trapped in the pulmonary capillary bed and their distribution,
which reflects the distribution of lung blood flow, is recorded
with an external photoscanner. The perfusion lung scan is an
important test because it is safe, readily available, essentially
noninvasive, and, if entirely normal, rules out a diagnosis
of PE. Ventilation scanning is performed with the use of radioactive
aerosols that are inhaled and exhaled by the patient while a
gamma camera records the distribution of the radioactivity in
the alveolar spaces.
An abnormal perfusion lung scan by itself is nonspecific and
seen in a variety of cardiorespiratory disorders. By combining
perfusion and ventilation scanning, certain patterns occur that
can be used to assign probabilities of PE. In general, the probability
of PE is reflected in the size and pattern of perfusion defects.
Thus, large defects are more likely to be caused by PE than
small defects, and mismatched defects (abnormal perfusion and
normal ventilation) are more likely to be caused by PE than
are matched defects. However, these distinctions are not absolute.
Thus, between 30% and 40% of patients with large perfusion defects
with a matching ventilation defect have PE, and a small mismatched
defect may not be diagnostic of PE.
Patients with subsegmental perfusion mismatches have a probability
of PE of ~40%, and those with subsegmental matches have a probability
of ~25%. The probability is lower in patients in whom clinical
suspicion of PE is low.
A high clinical probability of PE combined with a high-probability
lung scan pattern is associated with PE in 96% of patients.
A moderate clinical probability combined with a high-probability
lung scan pattern is positively associated with PE in 80% to
88% of cases. In most circumstances, the presence of these combinations
of clinical probabilities and lung scan findings can be used
to make a clinical decision to diagnose PE and treat the patient
accordingly. Unfortunately, these two combinations of clinical/lung
scan patterns (ie, a high-probability lung scan with a high
or moderate clinical probability) occur in only 12% to 32% of
patients with abnormal perfusion scans.3,123 In addition, only
~50% of patients with a high-probability lung scan but a low
clinical probability have PE.Although this combination is uncommon,
it is important, because it would be inappropriate to make a
diagnosis of PE without further investigation in this group.
If both clinical probability and lung scan probability are
low, then PE is very unlikely (occurring in <6% of patients),
and for practical purposes a diagnosis of PE can be excluded.123
This combination of clinical/lung scan pattern occurs in ~15%
of patients with an abnormal lung scan. Thus, a management decision
to either treat or not treat without further investigation can
be made in <50% of patients with clinically suspected PE
with an abnormal lung scan. In the remaining patients with suspected
PE and an abnormal perfusion scan, further investigations for
venous thrombosis or PE are required to either rule in or rule
out a diagnosis of PE. An approach to diagnosis of venous thrombosis
is shown in Fig 7.
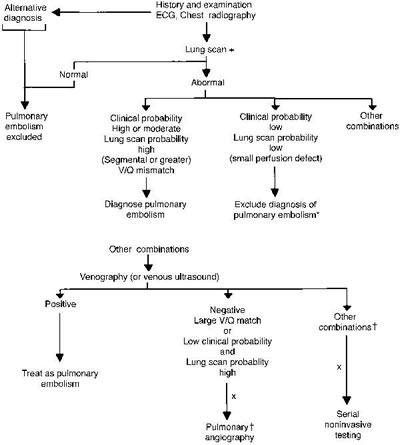
Fig 7. Diagnostic approach when pulmonary embolism is suspected.
*Can be followed with serial venous ultrasound. +Pulmonary angiography
may be preferable in a patient whose condition is unstable.
xBilateral venograms could be performed initially and proceed
only if results are negative. ?Other combinations include low
clinical probability and intermediate or indeterminant lung
scans and intermediate clinical probability and low-probability
lung scans.
COMPUTED TOMOGRAPHY SCANNING
Computed tomography scanning may reveal emboli in the main,
lobar or segmental pulmonary arteries with >90 percent sensitivity
and specificity. Accurate results have been reported for large
PE. However, for subsegmental emboli, the sensitivity and specificity
appear to be lower. The incidence of isolated subsegmental emboli
appears to be approximately 6 to 30 percent, with the former
figure likely being more representative. Of note, even with
the gold standard diagnostic test (arteriography), two referee
readers agreed on the presence or absence of subsegmental emboli
in only 66 percent of cases. Another study, using selective
pulmonary arteriography, indicated excellent agreement on main,
lobar, and segmental emboli but only 13 percent agreement on
subsegmental emboli . Thus, this apparent limitation with spiral
CT scanning is also a concern with angiography.
The incorporation of CT scanning into diagnostic algorithms
for PE is being endorsed increasingly. However, no prospective
multicenter randomized clinical trials large enough to unequivocally
prove the sensitivity and specificity of contrast-enhanced CT
scanning in patients with suspected PE have been performed.
Most have been singlecenter trials of moderate size. The value
of CT for large emboli appears clear, however.
Contrast-enhanced electron-beam CT also appears useful in diagnosing
acute PE. In one comparison with pulmonary angiography, only
8 of 720 vascular zones (1.1 percent) were considered inadequately
visualized with electron-beam CT. As with spiral CT, three-dimensional
reconstruction techniques can be applied to the pulmonary vessels
to better define vessels located within the plane that has been
sectioned. Another important advantage of these CT techniques
over the V/O scan is the concomitant ability to define nonvascular
structures such as airway, parenchymal, and pleural abnormalities,
lymphadenopathy, and cardiac and pericardial disease. Prospective
randomized clinical trials comparing these techniques with the
standard diagnostic approach to PE will help to determine their
precise role. It appears that CT scanning is being increasingly
utilized.
Victor F.Tapson,Md,Pulmonary Embolism,Hurst's
The Heart,10th Edition.2001,Pages1630-1631.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is also being utilized to evaluate
clinically suspected PE at some centers. One clinical trial
compared MRI with spiral CT: the average sensitivity of CT for
five observers was 75 percent and of MRI 46 percent. The average
specificity of CT was 89 percent, compared with 90 percent for
MRI. Sensitivity and specificity values for expert readers were
higher, however. Spiral CT may be somewhat more useful than
MRI for detecting PE at the present time, but MRI has several
attractive advantages, including excellent sensitivity and specificity
for the diagnosis of DVT. As in the case of CT scanning, the
diagnosis of entities other than PE using MRI is a major advantage
over the V/O scan.
Diagnostic algorithms for patients presenting with suspected
DVT and PE have been recommended in the American Thoracic Society
Consensus Statement and allow for a certain degree of flexibility
with regard to specific diagnostic modalities utilized.
Victor F.Tapson,Md,Pulmonary Embolism,Hurst's
The Heart,10th Edition.2001,Pages1630-1631.
Approach to Treatment
The objectives of treating venous thrombosis and PE are to
prevent local extension of the thrombus, prevent the thrombus
from embolizing, and, in certain clinical circumstances, accelerate
fibrinolysis. Anticoagulants are effective in most patients
for preventing clinically important local extension of thrombosis,
but they must be continued for weeks to months after the acute
event and they may not prevent long-term complications of thrombosis.
Of the two anticoagulants in current use, heparin acts immediately
by catalyzing the inhibition of activated coagulation factors
(principally thrombin and factor Xa) by antithrombin III (AT-III),
while coumarins act much more slowly by inhibiting synthesis
of fully gamma-carboxylated vitamin K-dependent coagulation
proteins. Both classes of anticoagulants inhibit the generation
of factor Xa and thrombin when administered in relatively low
doses. Oral anticoagulants do not inhibit thrombin activity
directly but modulate further thrombin generation by lowering
functional coagulation factors that participate in positive
feedback loops. Heparin can inhibit thrombin activity as well
as further thrombin generation by modulating positive feedback
loops.
Low concentrations of heparin can inhibit the early stages
of blood coagulation, but higher concentrations are needed to
inhibit the much higher concentrations of thrombin that are
generated if the coagulation process resists modulation. If
fibrin is formed, even higher concentrations of heparin are
required to modulate the procoagulant effects of clot-bound
thrombin, which is site-protected from inhibition by heparin/AT-III
and can provide an ongoing procoagulant stimulus in the vicinity
of the clot. Some of the new anticoagulants, including hirudin
and its fragments, are effective inhibitors of clot-bound thrombin
and may therefore be more effective than heparin in neutralizing
the procoagulant effects of the fibrin-bound thrombin.
The fibrinolytic enzymes streptokinase, urokinase, and TPA
accelerate the rate of dissolution of thrombi and emboli. Thrombolysis
is more expensive than anticoagulant therapy and is associated
with a higher risk of bleeding, so its use should be restricted
to patients who are likely to benefit from it. Two types of
patient groups have the potential to benefit from thrombolytic
therapy: those with major PE and selected patients with major
venous thrombosis. Surgical removal of the thrombus (venous
thrombectomy) or the embolus (pulmonary embolectomy) is rarely
indicated. In patients with venous thrombosis, PE can be prevented
very effectively with anticoagulant therapy. Pulmonary emboli
can also be prevented by inserting a filter into the vena cava,
but this approach is used only if anticoagulant therapy is contraindicated
because of bleeding or if PE has recurred despite adequate treatment
with anticoagulants (see below for definition of adequate anticoagulant
therapy).
There is good evidence that patients with PE have a high mortality
and a high rate of recurrence if untreated. There is also good
evidence that patients with symptomatic proximal or calf vein
thrombosis have a high recurrence rate without treatment. Anticoagulation
reduces mortality and recurrence in patients with acute PE and
reduces recurrence in patients with DVT.
Use of Anticoagulant Therapy
A working approach to the use of anticoagulants is described
below. A more comprehensive guide, "Guidelines to Anticoagulant
Therapy," (Circulation 1994;89:1449-1489) is available
in reprints from the Office of Scientific Affairs, American
Heart Association, 7272 Greenville Ave, Dallas, TX 75231-4596.
(Telephone 800-242-8721.)
Heparin
Heparin should be initiated with an intravenous bolus of 5000
U followed either by an intravenous infusion of 1400 U/h or
a subcutaneous injection of ~17 500 U twice daily. A weight-adjusted
dose regimen can also be used. This regimen consists of a continuous
intravenous infusion in a bolus dose of 80 U/kg followed by
an infusion at 18 U/kg per hour. The aPTT should be performed
~6 hours after the bolus and initiation of the continuous infusion
and at least daily thereafter to maintain the aPTT in the therapeutic
range equivalent to an anti-factor Xa heparin level of 0.3 to
0.7 U/mL. Warfarin can be started within the first 24 hours.
Heparin is continued for 5 days150,151 or longer until prothombin
time (PT) has been in the therapeutic range for a minimum of
2 consecutive days. It is essential that the initial dose of
heparin be adequate to achieve a therapeutic aPTT and that the
period of overlap of heparin and warfarin is sufficient to allow
the full antithrombotic effects of warfarin to be expressed
( Table 6).
Table 6. Guidelines for Use of Anticoagulants
| Bolus dose of heparin: 5000 U IV |
| Initial maintenance dose of heparin: 32 000 U IV per 24
h by continuous infusion or 17 000 U subcutaneously to be
repeated after adjustment at 12 h |
| Adjust dose of heparin at 6 h according to nomogram. Maintain
aPTT in therapeutic range |
| Repeat aPTT 6 times every hour until in therapeutic range
and then daily (see nomogram |
| Start warfarin 10 mg at 24 h and 10 mg next day. |
| Overlap heparin and warfarin for at least 4 d.
|
| Perform PT daily and adjust warfarin dose to maintain
INR at 2.0 to 3.0.
|
| Continue heparin for a minimum of 5 d, then stop if INR
has been in therapeutic range for at least 2 consecutive
days.
|
| Continue warfarin for 3 mo and monitor PT daily until
in therapeutic range, then 3 times during first week, twice
weekly for 2 wk, or until dose response is stable, and then
every 2 wk.
|
Obtain a pretreatment hemoglobin level, platelet count,
PT, and aPTT and repeat platelet count daily until heparin
stopped.
|
*See text for modified recommendations for iliofemoral thrombosis
and major pulmonary embolism.
aPTT indicates activated partial thromboplastin time; PT, prothrombin
time; INR, International Normalized Ratio.
The distinction between expression of the anticoagulant and
antithrombotic effects of warfarin is discussed in a subsequent
section of this report.
Therapeutic Range
The concept of a therapeutic range is based on experimental
studies in animals and subgroup analysis of the results of two
prospective studies in humans. The animal studies demonstrated
that prevention of growth of experimental venous thrombi required
doses of heparin that prolonged the aPTT to approximately twice
that of control subjects. These doses were equivalent to a heparin
level of 0.2 U/mL by protamine titration of the thrombin time.
In the clinical studies, comparisons of the rates of recurrence
between patient subgroups demonstrated that risk of recurrence
was increased if the aPTT ratio was less than 1.5 times the
mean of the normal range.
The results of these studies have led to the recommendation
that the therapeutic range of heparin should be an aPTT ex vivo
(ie, measured on plasma of patients treated with heparin), which
is equivalent to a heparin level by protamine titration of the
thrombin time of 0.2 to 0.4 U/mL or an anti-factor Xa heparin
level of 0.3 to 0.7 U/mL. For many commercial aPTT reagents,
the therapeutic range is ~1.8 to 3.0,98 although for less sensitive
reagents it is 1.5 to 2.0 ( Table 7).
Table 7. Therapeutic Range for Heparin
| aPTT |
~1.5-3.0 times mean of laboratory normal range |
| Heparin level: thrombin/protamine titration |
0.2-0.4 U/mL |
| Heparin level: Antifactor Xa |
0.3-0.7 U/mL |
*Depends on sensitivity of aPTT reagents to heparin.
aPTT indicates activated partial thromboplastin time.
A large between-patient variation in dosage is required to
achieve a therapeutic aPTT response in patients with VTE.
With a continuous infusion of heparin started at a dose of
32 000 U per 24 hours after a bolus of 5000 U, approximately
one third of patients are below the therapeutic range at 6 hours,
one third are in the therapeutic range, and one third are above
the therapeutic range. By adjusting the dose according to a
specially developed dose-adjustment nomogram ( Table 8)
Table 8. An Intravenous Heparin-Dose Nomogram
Based on aPTT Drawn 6 Hours After Starting Heparin*
| |
|
Stop |
Rate |
Rate |
|
| |
Bolus |
Infusion |
Change |
Change |
Repeat |
| aPTT (s) |
Dose |
(min) |
(mL/h)† |
(U/24 h) Repeat |
aPTT |
| <50 |
5000 U |
0 |
+3 |
2880 |
6 h |
| 50-59 |
0 |
0 |
+3 |
2880 |
6 h |
| 60-85? |
0 |
0 |
-2 |
1920 |
Next AM |
| 86-95 |
0 |
0 |
-2 |
1920 |
Next AM |
| 96-120 |
0 |
30 |
-4 |
3840 |
6 h |
| >120 |
0 |
60 |
-4 |
3840 |
6 h |
*As recommended in Table 6.
?When infusion fluid 1 mL/h=40 U/h (ie, 20 000 U heparin in
500 s).
?aPTT equivalent to heparin level of 0.3-0.7 U/mL by antifactor
Xa assay.
aPTT indicates activated partial thromboplastin time.
in which the aPTT response is obtained every 6 hours until
the therapeutic range has been achieved, more than 80% of patients
are within the therapeutic range at 24 hours and more than 90%
are within this range at 48 hours.
The anticoagulant effect of heparin is influenced by its nonspecific
binding to plasma proteins that compete with AT-III for heparin
binding and by the rate of heparin clearance. Many of the heparin-binding
proteins are acute-phase reactants that are elevated to a variable
degree in sick patients. The elevated levels of these plasma
proteins contribute to heparin resistance seen in sick patients,
and the variable concentrations of these binding proteins contribute
to the differences in anticoagulant response among patients.
One of these acute-phase reactant proteins, factor VIII, also
reduces the effect of heparin on the aPTT; thus, in many sick
patients the observed resistance to heparin and variability
in dose response is greater when monitored with an aPTT than
with an anti-factor Xa heparin assay or a thrombin clotting
time.Differences in the rates of heparin clearance between patients
also contribute to interindividual variability in patients'
responses.
Treatment of patients who fail to achieve an adequate aPTT
response despite high doses of heparin has been clarified by
the results of a randomized trial. Patients with venous thrombosis
whose aPTT response to high doses of heparin (more than 35 000
U per 24 hours) was subtherapeutic were randomly allocated to
monitoring with either aPTT or a heparin level of 0.3 to 0.7
anti-factor Xa units. These therapeutic ranges (for both methods
of monitoring) correspond to a heparin level by thrombin time
protamine titration of 0.2 to 0.4 U/mL. Many patients who had
subtherapeutic aPTT values had heparin levels >0.3 U/mL.
In patients randomly assigned to monitoring by aPTT, the dose
of heparin was increased until the test result was in the therapeutic
range. Despite receiving a lower dose of heparin, patients randomly
assigned to monitoring by heparin level had a low rate of recurrence
that was no different than the group randomly assigned to monitoring
with aPTT. Heparin assays based on an anti-factor Xa assay or
a thrombin time should be used to monitor heparin therapy in
patients who have a long aPTT due to a "lupus anticoagulant."
The targeted therapeutic range should be 0.3 to 0.7 anti-factor
Xa units or 0.2 to 0.4 U/mL by protamine titration of the thrombin
time.
Heparin Assays
Heparin assays using a chromogenic substrate are easy to perform
in any clinical laboratory, although they are not often available
clinically. Heparin assays are more expensive than aPTT assays;
therefore, it is recommended that their use be limited to the
10% to 20% of patients whose aPTT response is below the lower
limit of the therapeutic range with heparin doses of 40 000
U per 24 hours.
In patients with an inadequate response to heparin therapy
by both the aPTT and heparin assay, the dosage of heparin is
increased, and an assay for AT-III is obtained. If the AT-III
level is <50% of normal, the patient is treated with infusions
of plasma or AT-III concentrate to elevate the AT-III level.
However, if the AT-III level is above 60%, the dose of heparin
is increased with the use of a heparin dose-adjustment nomogram.
Duration of Heparin Therapy
The practice of a 7- to 10-day course of heparin therapy has
been changed because of the findings of two randomized studies
performed in patients with DVT. The studies reported that a
4- to 5-day course of heparin was as effective as a 9- to 10-day
course of heparin. The results of these two studies have important
practical implications because the shorter course of heparin
facilitates early discharge of patients from the hospital.
Although the findings of these studies can likely be generalized
to most patients, they may not be applicable to patients with
large iliofemoral vein thrombosis or major PE, because these
two classes of patients were excluded from one study150 and
formed only a small proportion of patients in the second.151
It is our practice to treat patients with large iliofemoral
vein thrombi and those with major PE with a 7- to 10-day course
of heparin and to delay starting warfarin therapy until the
aPTT has been in the therapeutic range for 3 days. The delay
in starting warfarin is used to ensure that patients receive
an adequate dose of heparin for at least 5 days.
Subcutaneous Heparin
The relative efficacy and safety of heparin administered by
subcutaneous and continuous intravenous infusion have been compared
in randomized trials. These studies demonstrate that the two
methods are equally safe and effective, provided that heparin
is given in an adequate starting dose and that the dose is adjusted
according to the aPTT ( Table 9).
Table 9. Pooled Analysis Randomized Trials of
Continuous Intravenous Versus Subcutaneous Heparin
| Trial |
No. of
Patients |
PE |
Fatal PE |
Major
Bleeding |
| Continuous intravenous |
437 |
8 (1.8%)
(0.57-3.1) |
1 (0.23%) |
21 (4.8%)
(2.8-6.8) |
| Subcutaneous |
441 |
12 (2.7%)
(1.2-4.2) |
1 (0.23%) |
17 (3.9%)
(2.1-5.6) |
PE indicates pulmonary embolism.
However, there is a clinically important reduction in the bioavailability
of heparin when administered subcutaneously in doses up to 15
000 U twice daily that results in subtherapeutic anticoagulant
and antithrombotic effects in a large percentage of patients.
On the other hand, there is good evidence from one large study
that heparin administered subcutaneously is both safe and effective
when started at a dose of 17 500 U twice daily after an intravenous
bolus of 5000 U. The dose is then adjusted according to the
aPTT. The aPTT is performed 6 hours after the morning injection
and the dose is then adjusted to maintain the midinterval aPTT
at 1.5 to 3.0 times the control value. Dose estimation is a
little more difficult than with continuous infusion, but the
feasibility of this approach has been demonstrated in a number
of clinical trials. Subcutaneous administration is difficult
in patients in shock or heart failure because of poor and variable
subcutaneous tissue blood flow.
Low-Molecular-Weight Heparins
Administration of LMWHs in a fixed dose by subcutaneous injection
has been compared with administration of dose-adjusted heparin
by continuous infusion for treatment of venous thrombosis. The
results, which have been summarized in a meta-analysis,indicate
that LMWHs are at least as effective and safe as standard heparin.
These findings raise the possibility that selected patients
with venous thrombosis might be suitable candidates for treatment
at home, an advance that would reduce cost and improve patient
convenience.
Like heparin, LMWHs do not cross the placental barrier, and
descriptive studies suggest they might be safe and effective
in pregnancy. In a randomized trial LMWHs were associated with
a much lower incidence of heparin-induced thrombocytopenia than
heparin and a lower incidence of osteoporosis.
Oral Anticoagulants
The need for oral anticoagulants after an initial course of
heparin is based on the results of two randomized studies that
demonstrated that the incidence of out-of-hospital recurrences
could be markedly reduced if heparin therapy was followed by
a 3-month course of warfarin. In one study in which the dose
of warfarin was adjusted to obtain an INR of 3.0 to 4.5, the
incidence of bleeding was very high. Another study was then
conducted in which patients with proximal vein thrombosis were
randomly assigned to treatment with either high- (INR, 3.0 to
4.5) or moderate-intensity (INR, 2.0 to 3.0) warfarin after
an initial course of heparin therapy. The incidence of recurrence
was equally low in both groups, but bleeding was approximately
four times higher in the high-intensity group. Based on the
results of this study, and subsequent experience with other
prospective clinical studies, the recommended therapeutic range
is an INR of 2.0 to 3.0.
An INR of 3.0 to 4.0 has been recommended for patients with
antiphospholipid antibodies, although there is some disagreement
on this issue.
Antithrombotic Effect of Warfarin
Warfarin therapy is usually monitored by prothrombin time (PT),
a test that is responsive to reduction of 3 of the 4 vitamin
K-dependent procoagulant clotting factors (factors II, VII,
and X). The conventional view is that the antithrombotic effect
of warfarin is reflected by its anticoagulant effect as measured
by PT. However, this view may not be correct during the induction
phase of warfarin therapy. During the first few days of warfarin
therapy, PT primarily reflects the reduction of factor VII activity,
which has a half-life of only ~6 hours, which is similar to
the half-life of the natural anticoagulant protein C. Subsequently
PT is prolonged by depression of factors X and II (prothrombin).
Therefore, for the first 24 hours of warfarin therapy there
is potential for a transient hypercoagulable state, resulting
from a reduction of levels of protein C before the effects of
warfarin on the activities of factors X and II are fully expressed.
There is evidence that reductions of factor II and, possibly,
factor X are more important than reduction of factors VII and
IX for the antithrombotic effect of warfarin. The evidence supporting
this view comes from the following observations. First, the
experiments of Wessler and Gitel, performed more than 40 years
ago with a stasis model of thrombosis in rabbits, showed that
the antithrombotic effects of warfarin require 6 days of treatment,
whereas the anticoagulant effect of warfarin as reflected by
prolongation of PT is seen within 2 days. These findings are
consistent with an explanation that the antithrombotic effect
of warfarin requires a reduction in activity of factor II, which
has a half-life of ~60 hours. Second, in more recent experiments
in a rabbit model of tissue factor-induced intravascular coagulation,
Zivelin et al demonstrated that the protective effect of warfarin
primarily reflects its ability to lower factor II levels. Thus,
selective infusion of factor II, and to a lesser extent factor
X, abolished the protective effects of warfarin in this model.
In contrast, infusion of factor VII or IX had no effect.
The concept that the antithrombotic effect of warfarin reflects
its ability to lower factor II levels provides a rationale for
overlapping heparin with warfarin in treatment of patients with
thrombotic disease until the factor II level is lowered into
the therapeutic range. Given that factor II has a half-life
of ~60 hours, an overlap of at least 4 days is necessary.
Optimal Duration
Patients with VTE are usually treated with oral anticoagulants
for 3 to 6 months. Shorter courses of oral anticoagulant therapy
have been investigated in randomized trials, but the results
have been inconclusive.It is now clear that risk of recurrence
varies in different subgroups. The risk of PE in patients with
isolated calf DVT is very low. There also is evidence, that
risk of recurrence is less in patients with a temporary or reversible
risk factor (eg, thrombosis secondary to surgery or trauma)
than it is in those with a continuing risk factor (such as associated
malignancy) or with idiopathic DVT (thrombosis in the absence
of a recognized risk factor). Prandoni and associates reported
that in patients with proximal vein thrombosis treated with
oral anticoagulants for 3 months, the rate of recurrent VTE
was 24% over 80 weeks in patients with idiopathic venous thrombosis
compared with 4.8% in those with a reversible risk factor. A
similar observation was made by Levine and associates.46 In
301 patients with proximal DVT given 3 months of warfarin and
then followed for an additional 9 months, there were 26 recurrent
thromboembolic events in 212 patients (12.3%) with either continuing
risk factors or idiopathic DVT, compared with 0 in 89 patients
with a transient reversible risk factor (P=.0007). None of the
recurrences were fatal. Thus, patients with an identifiable
reversible risk factor (such as surgery) appear to respond well
to a 6-week to 3-month course of therapy, whereas patients without
a reversible risk factor have a high incidence of recurrence
despite 3 months of oral anticoagulation.
Similar findings have been reported in two randomized studies.
In the first report, 712 patients with DVT and PE were randomly
assigned to either 4 or 12 weeks of anticoagulant therapy. The
rate of recurrent VTE was 7.8% in patients treated for 4 weeks
and 4.0% in those treated for 12 weeks. Only 1 of 116 patients
(0.86%) with postoperative VTE had a recurrent event; whereas
among the 506 "medical" patients, 4.0% of patients
treated for 12 weeks and 9.1% of patients treated for 4 weeks
experienced a recurrence. Only 1% of all patients had fatal
PE. These results suggest that a short course of anticoagulation
might be adequate for patients with postoperative thrombosis,
but a longer course of treatment is necessary for patients without
a reversible risk factor. In a more recent study, 897 patients
with a first episode of DVT or PE were treated with at least
5 days of heparin or LMWH and randomly allocated to receive
6 weeks or 6 months of warfarin, with the goal of reaching an
INR of 2.0 to 2.85. The incidence of recurrence over 2 years
of follow-up was 18.1% in the 443 patients who received 6 weeks
of oral anticoagulation compared with 9.5% in the 454 patients
who received 6 months of therapy (P<.001). However, as in
the other studies, the incidence of recurrent thromboembolism
was much lower in both groups in patients with reversible risk
factors.
The observed difference in recurrence rates between patients
with and without reversible risk factors is relevant to the
issue of optimal duration of oral anticoagulant therapy. Thus,
the low absolute incidence of thrombosis in patients with temporary
risk factors suggests that a short course of treatment might
be appropriate for the subgroup of patients with reversible
risk factors, whereas long-term anticoagulant therapy should
be considered for patients without a reversible predisposing
factor. At present, however, there is insufficient evidence
to support lifelong treatment for all patients with idiopathic
thrombosis. Instead, it would be reasonable to use anticoagulant
therapy for 6 weeks in patients with a reversible risk factor
and to continue anticoagulation for up to 6 months in patients
with idiopathic venous thrombosis. An indefinite duration of
anticoagulation should be considered in patients with venous
thrombosis associated with active malignant disease who are
often bedridden and receiving chemotherapy, which contributes
to their hypercoagulable state. Long-term anticoagulant therapy
should also be considered for patients who have multiple recurrent
episodes of idiopathic VTE and those with inherited thrombophilia
who have suffered one or more unprovoked episodes of major VTE.
Recommendations for Duration of Warfarin
Therapy
Patients with a first episode of VTE should be treated for
6 weeks to 3 months if they have a reversible risk factor and
for 3 to 6 months if they have idiopathic venous thrombosis.
Warfarin therapy should be continued for longer periods, possibly
for life, in patients with documented idiopathic thrombosis
who have 1 of the 4 inherited molecular abnormalities (deficiencies
of AT-III, protein C, protein S, or activated protein C resistance)
and in those who have a lupus anticoagulant or anticardiolipin
antibody, because these laboratory abnormalities predispose
them to recurrent venous thrombosis. Treatment of patients with
these blood abnormalities who develop venous thrombosis after
a well-recognized provocation (eg, surgery) is uncertain. Indefinite
anticoagulation might not be warranted, although some authorities
believe so. The AHA also recommends that patients who have more
than two documented episodes of recurrent venous thrombosis
and patients with at least one episode of thrombosis and active
cancer should be treated with anticoagulants indefinitely. Finally,
patients with ongoing risk factors (eg, immobilization in a
plaster cast) should be treated until the period of risk is
over.
Most patients requiring long-term anticoagulant therapy respond
well to warfarin targeted to an INR of 2.0 to 3.0. However,
some patients with cancer have a resistance to warfarin and
require long-term treatment with heparin, administered in full
doses by subcutaneous injection. The optimal intensity of anticoagulation
therapy is uncertain for patients with a lupus anticoagulant
or cardiolipin antibody who require long-term anticoagulation.
There are reports, based on retrospective analyses of observational
studies, that patients with the antiphospholipid antibody syndrome
and thrombosis are inadequately protected from recurrent episodes
of VTE if treated at a targeted INR of 2.0 to 3.0. In contrast,
a recent smaller prospective study in lupus anticoagulant-positive
patients with venous thrombosis but without other manifestations
of the antiphospholipid antibody syndrome reported that these
patients with fewer complications respond well to warfarin at
an INR intensity of 2.0 to 3.0. It is uncertain whether the
discrepant findings reported in these studies result from differences
in patient populations or differences in the responsiveness
of PT reagents to the lupus anticoagulant in patients who receive
anticoagulation with warfarin. Thus, it is possible that with
some PT reagents the INR result is artifactually prolonged by
the lupus anticoagulant and therefore does not reflect the true
anticoagulant effects of warfarin.
Thrombolytic Therapy
Thrombolytic therapy is more effective than heparin in producing
rapid lysis of thromboemboli. However, it is more expensive
than heparin, it is associated with a higher risk of bleeding,
and it is not indicated in most patients with PE because they
do well clinically with anticoagulant therapy. It is contraindicated
in the postoperative period and in other situations in which
there is a high risk of bleeding. Thrombolytic therapy has lifesaving
potential for patients with massive PE and should be considered
in patients with major PE who have syncope, hypotension, severe
hypoxemia, or heart failure. Thrombolytic therapy should also
be considered for patients with a submassive embolism and underlying
cardiac or respiratory disease. Limited evidence suggests that
thrombolytic therapy prevents postthrombotic syndrome in some
patients with acute venous thrombosis of recent onset. Thrombolytic
therapy may also be indicated in selected patients (both young
and old without risk factors for bleeding) with extensive proximal
vein thrombosis.
Caval Interruption
Although anticoagulation is the standard treatment for acute
venous thrombosis and PE, venous interruption procedures may
be indicated for VTE when anticoagulation is ineffective or
unsafe. The most common indication for venous interruption in
patients with DVT or PE is anticoagulant-induced bleeding or
anticipation of hemorrhagic complications in a patient with
a predisposing lesion, such as a bleeding peptic ulcer, gastrointestinal
malignancy, recent intracranial operation, or an underlying
hemorrhagic state (eg, liver failure or thrombocytopenia). The
second indication for venous interruption is failure of anticoagulation,
provided that the anticoagulant effect has been within the prescribed
therapeutic range (an aPTT corresponding to an anti-factor Xa
heparin level >0.3 U/mL or an INR >2.0). Development of
new PE or substantial extension of venous thrombosis should
be documented by objective tests before recurrent thromboembolism
is accepted as a diagnosis, because new symptoms in a patient
with an established venous thrombosis of PE are often misinterpreted
as evidence for recurrence in a patient receiving anticoagulation.
Other indications for venous interruption are more controversial.
These include
Major PE with severe cardiovascular instability
As an adjunctive procedure in patients who undergo pulmonary
embolectomy
Prophylactic interruption of the inferior vena cava in patients
at exceptionally high risk of VTE, particularly if there is
a relative contraindication to anticoagulation
Intracaval Devices
The first intracaval device to be widely used was the inferior
vena caval umbrella devised by Mobin-Uddin et al.The umbrella
filter is inserted through a cutdown in the internal jugular
vein and passed under fluoroscopic control through the superior
vena cava and right atrium into the inferior vena cava, where
its position below the renal veins is confirmed by phlebography.
When it is expelled from its capsule applicator, the pointed
struts engage the wall of the cava and hold the filter in place.
The device contains fenestrations to maintain venous blood flow.
The results following implantation of 4699 filters during the
first 6 years after the umbrella filter became available were
summarized by Mobin-Uddin.The initial design had a diameter
of 23 mm and was associated with proximal migration in 27 of
2848 applications (0.9%). The frequency of proximal migration
was reduced to 0.4% by increasing the diameter to 28 mm. Complete
occlusion of the filter occurred in 30% to 45% of patients due
to thrombosis around the device or trapping of an embolus.190,191
The reported rate of recurrent PE was 12%. Less common complications
included perforation of adjacent organs (eg, duodenum or ureter)
and breakage.
The Greenfield filter has essentially replaced the Mobin-Uddin
umbrella. The filter, which resembles an umbrella consisting
only of struts, is placed with its apex directed proximally.
With this design, emboli are retained in the center of the cone,
where the spokes are closer together and the trapping efficiency
greater. The central positioning of entrapped emboli facilitates
blood flow past the trapped embolus and may encourage fibrinolysis,
thereby accounting for the high rate of patency with this device
(95%). Magnant and coworkers reviewed the experience with placement
of the Greenfield filter. They concluded that percutaneous placement
of inferior vena caval filters had supplanted operative placement
and that no major morbidity had been associated with use of
the Greenfield filter. The bird's nest filter was invented by
Roehm and described in 1984. When compared directly with the
Greenfield device, the bird's nest filter appeared to be more
readily dislodged and more easily subjected to local thrombosis.
Occlusion of the cava by a balloon has been proposed by Hunter
et al196 and Moser et al.197 The balloon is inserted as a percutaneous
procedure. A potential advantage of balloon occlusion is that
caval obstruction can be temporary; once the threat of embolization
has subsided, the balloon can be deflated and removed. However,
thrombosis can occur around the balloon. In a report of up to
18 years of experience involving 191 cases, Hunter and associates198
reported no malfunction of the inflation mechanism and no migration
from the site of inflation. No patients had recurrent PE after
balloon inflation. In 39% of patients, the legs appeared normal
and free of edema.
Surgical Removal
Thrombectomy for acute venous thrombosis and pulmonary embolectomy
for acute PE to relieve acute obstruction are rarely used. Thrombectomy
is of limited benefit because it is usually complicated by acute
recurrence despite postoperative anticoagulant therapy; it leaves
a de-endothelialized venous surface that is highly thrombogenic.
However, it is indicated to rapidly reduce venous obstruction
in patients with phlegmasia cerulea dolens with impending venous
gangrene. Although pulmonary embolectomy can be a lifesaving
procedure in a patient with massive embolism, most hospitals
do not have the resources, personnel, or facilities for this
type of surgery. Furthermore, most patients who are likely to
benefit from pulmonary embolectomy die before they can be diagnosed
and treated,201 and some candidates for emergency pulmonary
embolectomy survive and do well with medical therapy. On the
other hand, elective pulmonary thromboendarterectomy can be
very effective and lifesaving in selected patients with chronic
large-vessel thromboembolic pulmonary hypertension.This operation,
which has been available for years but abandoned by many centers
because of a high postoperative mortality, has been revived
by the work of the San Diego group.The success of the procedure
is highly dependent on the availability of a skilled and experienced
team of surgeons and internists.
Upper-Extremity DVT
The frequency of upper-extremity venous thrombosis involving
the axillary and/or subclavian veins has increased in the last
decade with the increasing use of long-term indwelling catheters.
Upper-extremity venous thrombosis is classified as primary and
secondary. Primary upper extremity thrombosis can be caused
by local venous compression produced by unusual movements or
positions of the arm ("effort thrombosis"), whereas
secondary thrombosis is usually caused by indwelling intravenous
devices. Effort thrombosis has been described following weight-lifting,
pole-vaulting, racquet sports, or direct and prolonged pressure
to the axilla. Axillary vein thrombosis can also be a manifestation
of the thoracic inlet syndrome or can be caused by direct trauma
or compression by tumor.206 Axillary or subclavian vein thrombosis
has been described in AT-III deficiency, protein S deficiency,
hypoplasminogenemia, and antiphospholipid syndrome. Thrombosis
secondary to the use of long-term indwelling catheters, often
used in administration of chemotherapy, is now a much more common
cause of upper-extremity thrombosis than effort thrombosis.
Upper-extremity venous thrombosis can be complicated by PE
and rarely by massive PE. The most important complications are
long-term disability caused by venous hypertension and loss
of venous access in patients requiring long-term chemotherapy.
Venous hypertension can produce swelling, fatigability, aching,
and weakness of the affected arm, particularly following activity.
The symptoms can be disabling in athletes or manual laborers
during and after activity involving the affected arm. The reported
frequency of disabling upper-extremity venous hypertension after
spontaneous axillary/subclavian vein thrombosis varies from
25% to 47%. Lower rates (12%) have been reported in a series
of patients treated with thrombolysis, but no randomized trials
have been reported comparing anticoagulants with thrombolysis.
The diagnosis of upper-extremity vein thrombosis is usually
suspected on clinical grounds and confirmed by venography. Optimal
visualization of the thrombosed axillary/subclavian veins is
best achieved by injecting the radiographic contrast into the
median basilic vein. Injection of contrast material into a distal
vein in the hand or wrist will demonstrate an obstruction and
the presence of collateral vessels but does not usually outline
the thrombus. Imaging studies in which color flow duplex ultrasound
was used lack the sensitivity of venography for upper-extremity
thrombosis.218
Various treatments have been advocated for primary upper-extremity
thrombosis. Resolution of acute symptoms can usually be obtained
with either anticoagulant or lytic agents. Anticoagulant therapy
is not usually associated with anatomic resolution of the thrombus
and clinical improvement because collaterals develop and bypass
the obstruction. Thrombolytic therapy appears to be more effective
than anticoagulants in producing early resolution. Local therapy
administered through a small catheter introduced through the
basilic vein and advanced into the clot has been advocated.
A loading dose of 250 000 IU urokinase infused into the clot
over 1 hour and then continued at a lower dose of 1000 IU/min
for up to 24 hours has been used successfully. The patient is
then treated with heparin for 5 days, followed by warfarin for
3 months. Surgical removal of the first rib has been advocated
by some if symptoms of venous obstruction persist after a course
of conservative treatment. However, the effectiveness of this
invasive approach has never been evaluated in an appropriately
designed clinical trial.
Long-term venous access through a central venous catheter is
required for treatment of long-term disorders requiring chemotherapy,
antibiotics, or hyperalimentation. Thrombosis of the subclavian/axillary
vein is a common complication of central venous catheterization.
These thrombi may be asymptomatic, although spontaneous resolution
is uncommon when long-term venographic follow-up studies are
performed.
The standard treatment of secondary axillary/subclavian vein
thrombosis has been removal of the catheter, limb elevation,
and anticoagulation. This approach usually results in rapid
improvement of symptoms, but on follow-up 70% of patients have
been reported to have some pain and/or swelling in the affected
arm. Of greater importance, the venous lumen is obliterated
and cannot be used again for venous access. Thrombolytic therapy
has been used successfully to treat secondary upper-extremity
thrombosis.Initial reports used high-dose systemic therapy.
More recently local catheter-directed thrombolytic therapy has
been used with apparent success.
A dosage regimen of urokinase has been established, empirically
consisting of 250 000 IU/h for 2 hours followed by 60 000 IU/h
until clot lysis has been achieved. Heparin can be given in
full doses either during or after completion of thrombolytic
therapy and anticoagulation with heparin, followed by warfarin
for ~3 months. With this approach, a 78% lysis rate has been
reported in a small study of 31 patients.229 Successful lysis
is more common with fresh thrombi.230
Diagnosis of Venous Thromboembolism in the Pregnant Patient
The diagnosis of VTE during pregnancy is difficult because
leg pain and swelling are frequent and usually not due to DVT,
and performance of radiological procedures is a problem because
of the fear of exposing the fetus to radiation.
Deep Vein Thrombosis
As in the nonpregnant patient, venous ultrasonography is used
as the initial diagnostic test. If venographic confirmation
of an equivocal test result is required, a limited venogram
can be performed without risk to the fetus by covering the patient's
abdomen with a lead-lined apron. A limited venogram allows visualization
of the calf veins, popliteal vein, and most of the superficial
femoral vein but not the iliac vein. Therefore, a normal limited
venogram does not exclude iliac vein thrombosis.
Pulmonary Embolism
The diagnosis of PE in pregnancy is essentially the same as
in the nonpregnant patient, with three exceptions designed to
avoid exposure of the fetus to ionizing radiation: (1) Ventilation
and perfusion scanning are performed at 50% of the usual dose;
(2) pulmonary angiography, if indicated, should be performed
via the brachial route rather than the femoral route; and (3)
venography, if indicated, should be limited, with shielding
of the abdomen.
Chest radiography, perfusion, and ventilation lung scanning
are performed with a reduced dose of radioisotope for the perfusion
scan (1 to 2 MCi). If the perfusion scan is normal, PE is excluded;
if the lung scan indicates a high probability of PE, the diagnosis
is made and the patient is treated with anticoagulants. If the
scan is nondiagnostic, the patient is investigated for DVT by
IPG or duplex ultrasound; if the test results are abnormal,
the patient should be treated with anticoagulants. If the results
are normal, a pulmonary angiogram should be considered.
Management of Venous Thromboembolism During Pregnancy
An excellent review of this subject has been published, and
the recommendations outlined below follow this report, which
should be read for additional details.
Heparin
A recent critical review of the literature of heparin therapy
during pregnancy reported that, contrary to a previous report,
heparin therapy during pregnancy is safe for the fetus. The
conclusion is corroborated by a cohort study in which the rates
of premature birth, spontaneous abortion, stillbirth, neonatal
death, and congenital malformation were not significantly higher
in 100 pregnant women treated with heparin than in the normal
population.Because heparin does not cross the placenta, there
is no increased risk of bleeding for the fetus.
Warfarin
In the review cited previously, the pooled rate of adverse effects
associated with warfarin therapy was high (26.1%). Warfarin
exposure between 6 and 12 weeks of gestation can be associated
with warfarin embryopathy, which is characterized by stippled
epiphyses and nasal hypoplasia. In a study by Iturbe-Alessio
et al,236 10 of 35 term pregnancies in which warfarin was administered
between 6 and 12 weeks were associated with warfarin embryopathy.
This is likely to be an overestimate, and the true incidence
of warfarin embryopathy is likely to be ~5% of infants if maternal
exposure occurs between 6 and 12 weeks of gestation. Warfarin
embryopathy has not been reported with warfarin exposure outside
this time period. Central nervous system abnormalities, both
hemorrhage and malformations, have been reported after warfarin
exposure at any time during pregnancy, but the incidence is
very low.233,236 Therefore, heparin is the anticoagulant of
choice for treatment of VTE during pregnancy. If warfarin is
used, it should be restricted to the second and early third
trimesters and avoided between 6 and 12 weeks of gestation and
near term to avoid delivery of an anticoagulated fetus.
Treatment of Acute Deep Venous Thrombosis and Pulmonary Embolism
Heparin is usually initiated with an intravenous bolus of 5000
U followed by a maintenance dose administered as a continuous
intravenous infusion of 32 000 U per 24 hours to prolong the
aPTT into the therapeutic range (~1.8 to 2.5 times control for
most reagents) for 5 to 7 days. After the initial intravenous
dose of heparin, subcutaneous heparin should be administered
every 12 hours in doses adjusted to prolong a 6-hour postinjection
aPTT into the therapeutic range. The aPTT should be checked
regularly, because heparin requirements may vary as pregnancy
progresses. The patient should be monitored three times in the
first week and then at least weekly thereafter. Anticoagulant
therapy should be continued throughout pregnancy and for 4 to
6 weeks after delivery. If the episode of VTE occurs late in
pregnancy, anticoagulation should be continued for a total of
3 months after the episode.
Long-term Anticoagulant Therapy Before Pregnancy
Patients who receive long-term warfarin therapy before pregnancy
for DVT/PE or prevention of systemic embolism should be treated
with subcutaneous heparin every 12 hours throughout pregnancy
in doses adjusted to prolong the 6-hour postinjection aPTT to
~1.5 to 2.5 times control. Two options are available when patients
receiving long-term anticoagulant therapy decide to conceive.
The first is to switch the patient to heparin before conception.
This has the advantage of avoiding any exposure of the fetus
to warfarin but increases the duration of heparin exposure if
conception is delayed. The second option is to continue warfarin
and perform regular pregnancy tests when conception is attempted.
As soon as the pregnancy test result is positive, warfarin should
be stopped and heparin started. This is probably safe for the
fetus, provided warfarin is discontinued before 6 weeks of gestation.
As mentioned above, no cases of fetal embryopathy have been
described with warfarin exposure before 6 weeks of gestation.
Warfarin therapy can be resumed after delivery.
Previous Deep Venous Thrombosis and Pulmonary Embolism
The optimal treatment of pregnant patients with previous DVT/PE
is unknown because there are no large prospective trials to
provide reliable estimates of the incidence of recurrence during
pregnancy. Prophylaxis with standard heparin, 5000 U every 12
hours, is a reasonable approach and is associated with a very
low recurrence rate.235 Surveillance with weekly IPG or duplex
ultrasonography may be a reasonable alternative to heparin during
pregnancy.
Delivery and Postpartum
If the patient is receiving 5000 U of heparin every 12 hours
at term, heparin can be discontinued at onset of labor. No increase
in bleeding is anticipated with this approach. If adjusted-dose
heparin is being administered at term, some pregnant patients
can have a prolonged aPTT for as long as 20 hours after their
last dose of subcutaneous heparin.237 To overcome the potential
risk of a long aPTT at delivery, elective induction can be planned
and heparin therapy discontinued 24 hours before induction.
In patients considered to be at high risk for thrombotic complications,
an intravenous heparin infusion can be started after discontinuation
of subcutaneous heparin. Because the half-life of intravenous
heparin is short,238 heparin can be discontinued 4 to 6 hours
before delivery with the expectation that the aPTT will be normal
at time of delivery.
After delivery, heparin and warfarin should be restarted as
soon as hemostasis is obtained, and heparin can be discontinued
after an appropriate period of overlap. When administered to
the nursing mother, warfarin is safe for the breastfed infant.239,240
Other Therapeutic Modalities
There are very few reports on the use of thrombolysis during
pregnancy. As a general rule, pregnancy is a relative contraindication
to thrombolytic therapy, and its use should be restricted to
patients with massive PE.241,242 LMWHs do not cross the placenta
and have been used successfully during pregnancy.169,243,244
Their advantage over standard heparin is a more predictable
dose response and a longer half-life after subcutaneous injection,
which allows administration once daily without frequent monitoring.
Management of Venous Thromboembolism in Children
Venous thromboembolism in children is much less common than
in adults. In general, recommendations for antithrombotic therapy
have been extrapolated from those used for adults. However,
optimal dosing for antithrombotic therapy in children is likely
to differ from adults because the anticoagulant response to
antithrombotic agents is different.
Incidence
Incidence of DVT/PE in the adult population is ~2.5% to 5.0%.
In comparison, incidence of DVT/PE in the general pediatric
population is reported to be 0.07 per 10 000 and 5.3 per 10
000 hospital admissions.246-248 Other comparisons illustrating
the lower risk of DVT/PE during childhood are the <1% incidence
of clinically apparent DVT/PE after lower limb or scoliosis
surgery and the relative absence of DVT/PE in children with
congenital thrombophilias.
Clinical Features
Ninety-five percent of DVT/PE in pediatric patients occurs as
a complication of serious diseases such as prematurity, cancer,
trauma/surgery, and congenital heart disease. Congenital prethrombotic
disorders account for <10% of DVT/PE in children. Children
at greatest risk for DVT/PE are younger than 1 year or teenaged.
DVT in the lower extremities is the most frequent noncentral
venous line thrombotic complication in children.251 The clinical
presentations of DVT and PE are similar to those in adults.
Central Venous Lines
Forty percent of DVT in children and more than 80% in newborns
occurs in the upper venous system secondary to use of central
venous lines, which are employed for short-term intensive care
or long-term supportive care in children requiring total parenteral
nutrition or therapy for cancer. Central venous line-related
DVT requires repeat anesthesia for replacement and can be complicated
by PE; it can cause superior vena cava syndrome and chylothorax
and can obliterate the upper venous system and so lead to postthrombotic
syndrome in the upper extremities.
Treatment of Children
Children older than 2 months who have DVT or PE should be treated
with intravenous heparin (bolus 75 U/kg; initial maintenance
of 20 U/kg per hour) sufficient to prolong the aPTT to a range
that corresponds to an anti-factor Xa level of 0.3 to 0.7 U/mL.
Treatment with heparin should be continued for 5 to 10 days
and oral anticoagulation overlapped with heparin for 4 to 5
days. For many patients heparin and warfarin can be started
together and heparin discontinued on day 6 if the INR is therapeutic.
Heparin therapy should be used for a longer period for massive
PE or iliofemoral thrombosis.
Long-term anticoagulant therapy should be continued for at
least 3 months, with oral agents (initial dose 0.2 mg/kg per
day) to prolong PT to an INR of 2.0 to 3.0.
Either indefinite warfarin therapy with an INR of 2.0 to 3.0,
low-dose anticoagulant therapy (INR, <2.0), or close monitoring
should be considered for children with a second recurrence of
venous thrombosis or a continuing risk factor such as central
venous line, antithrombin deficiency, or protein C or S deficiency.
Newborns with a central venous line in place should be treated
with intravenous heparin in doses of 1 to 5 U/h through the
catheter.
Treatment of Newborns
The optimal regimen for anticoagulation therapy in treatment
of newborns with DVT, PE, or arterial thrombosis is uncertain.
If anticoagulation is indicated, a short course (10 to 14 days)
of intravenous heparin (75 U/kg bolus; maintenance 28 U/kg per
hour), sufficient to prolong the aPTT to an anti-factor Xa level
of 0.3 U/mL, should be used.
The role of thrombolytic agents in treatment of VTE is uncertain.
Further clinical investigation is needed before more definitive
recommendations can be made. If thrombolytic therapy is used,
either urokinase or TPA is preferable to streptokinase, and
supplementation with plasminogen may be helpful.
Complications of Anticoagulation
Treatment of patients who develop complications during anticoagulant
therapy involves management of the actual complication and subsequent
management of the thromboembolic event for which the patient
is being treated.
Bleeding is by far the most important complication of anticoagulant
therapy. The approach to bleeding depends on the severity of
bleeding, the anticoagulant and dose used, results of laboratory
tests at the time of bleeding, the length of time the patient
has been treated with anticoagulants, and the seriousness of
the thromboembolic event for which the patient is being treated.
Heparin
The frequency of clinically important bleeding during a 5-
to 10-day course of heparin therapy varies between 3% and 10%,
depending on whether the patient is at high or low risk. In
many cases bleeding is not life-threatening and does not require
discontinuation of heparin. Because heparin has a relatively
short circulating half-life (60 minutes), the anticoagulant
effect is reversed fairly rapidly after treatment is discontinued.
In most cases bleeding is treated by discontinuing heparin,
applying local pressure as appropriate, and replacing blood
if necessary.
If bleeding is potentially life-threatening (eg, intracerebral,
intraspinal, retroperitoneal, or severe gastrointestinal), heparin
should be stopped and the anticoagulant effect reversed with
protamine sulfate. Protamine sulfate is a strong basic substance
that rapidly neutralizes the effect of heparin. The appropriate
neutralizing dose depends on the dose of heparin and route and
time of administration. If protamine sulfate is used within
minutes of intravenous heparin injection, then a full neutralizing
dose, 1 mg protamine per 100 U heparin, should be given. Since
the half-life of heparin is ~60 minutes, only 50% of a full
neutralizing dose is required 1 hour after the last heparin
injection, and only 25% of the full neutralizing dose is required
after 2 hours.
Protamine sulfate can produce a hypotensive response if given
rapidly, so the dose should be injected slowly over a 20-minute
period. Some patients may also develop a hypersensitivity reaction
to protamine sulfate.
Heparin rebound may occur if very large doses of heparin are
given.280-283 Therefore, it may be necessary to repeat administration
of protamine if laboratory tests demonstrate a residual heparin
effect.A direct assay of heparin activity, thrombin time, or
aPTT should be performed both before and immediately after protamine
is infused, and the test should be repeated 2 hours later to
determine whether the neutralizing effect of protamine on heparin
is permanent or transient.
If bleeding occurs when the aPTT response is in the therapeutic
range or just beyond the therapeutic range, or if the anticoagulant-associated
bleeding is potentially life-threatening, treatment with anticoagulant
therapy should be stopped, and an alternative form of treatment
should be used to manage the thromboembolic event. If the patient
has proximal vein thrombosis or major PE, a caval interruption
procedure should be considered. If the patient has calf vein
thrombosis, the course of the thrombus can be monitored with
serial venous ultrasound imaging and a caval interruption procedure
used if thrombosis is extended.
The risk of bleeding is influenced by five variables: the patient's
clinical condition,the dose of heparin, the anticoagulant response,
method of administration, and concomitant use of aspirin or
thrombolytic agents.
The most important risk factor for bleeding is recent surgery
or trauma. Other risk factors are renal failure, old age, and
peptic ulcer disease. There is a relation between bleeding and
both heparin dose and anticoagulant effect. Bleeding is greater
when heparin is administered by intermittent intravenous injection.
Other Complications of Heparin Therapy
Other complications of heparin are thrombocytopenia, with or
without thrombosis; osteoporosis, which occurs only with long-term
treatment; and local skin hypersensitivity and skin necrosis
confined to subcutaneous injection sites. Other complications
are very rare and include anaphylaxis, hypoaldosteronism, and
alopecia. In addition, patients treated with heparin can develop
hyperkalemia and often develop an asymptomatic increase in plasma
levels of hepatic transaminases.
If a patient develops local skin reactions at the site of injection,
the source of heparin should be changed because local reactions
may not occur with a different preparation of heparin, including
LMWHs.
Thrombocytopenia
Thrombocytopenia is a well-recognized complication of heparin
therapy. Two forms of thrombocytopenia are described: an early
benign, reversible nonimmune thrombocytopenia and a late, more
serious IgG-mediated immune thrombocytopenia. The mechanism
of the early form, which is not associated with adverse clinical
sequelae, is uncertain but could be the result of direct weak
activation of platelets by heparin. The immune form of heparin-induced
thrombocytopenia is characterized by strong IgG-mediated platelet
activation and is associated with a substantial risk of thrombotic
complications.
The incidence of serologically confirmed heparin-induced thrombocytopenia
was investigated in a large clinical trial that compared unfractionated
heparin (7500 U twice daily) with LMWH (30 mg enoxaparin twice
daily) for prophylaxis after elective hip surgery.170 The incidence
of heparin-induced thrombocytopenia was ~1% at 7 days and ~3%
at 14 days in patients receiving unfractionated heparin and
0% in those receiving LMWH. Other prospective studies with higher
(therapeutic) doses of heparin have reported a similar incidence
of thrombocytopenia.
Heparin-induced thrombocytopenia usually begins between 5 and
15 days after the start of heparin therapy (median, 10 days),
but it has been reported within hours of starting heparin in
patients who have received heparin within the previous 3 months.
Thrombosis associated with heparin-induced thrombocytopenia
can be heralded by a fall in platelet count without overt thrombocytopenia
(eg, from 350 000 to 150 000). For this reason, patients who
receive heparin should undergo a platelet count daily, and if
the platelet count falls by 50% or more, heparin should be stopped
and an alternative management strategy instituted.
Thrombocytopenia and Paradoxical Thrombosis
Heparin-induced thrombocytopenia is a highly prothrombotic disorder.
In a large prospective study of heparin therapy after elective
hip surgery, risk for thrombosis was dramatically increased
(odds ratio, 37) in patients with heparin-induced thrombocytopenia,
compared with those who did not develop it. Although many case
series have emphasized the association of heparin-induced thrombocytopenia
with arterial thrombosis ("white clot syndrome"),
it is now clear that venous thrombosis is much more common with
heparin-induced thrombocytopenia than arterial thrombosis. Overall,
prospective studies suggest that thrombosis associated with
heparin-induced thrombocytopenia occurs in ~1% of patients who
receive unfractionated heparin for more than 5 days.
Bleeding complications have been described in patients with
heparin-induced thrombocytopenia, but they are less frequent
and much less important than thrombotic complications.
Laboratory Manifestations and Pathogenesis
Typically, the platelet count nadir in heparin-induced thrombocytopenia
is between 20 and 150 000 per milliliter (median nadir, 50 000).
Approximately 5% of patients have concomitant hypofibrinogenemia
associated with disseminated intravascular coagulation.The platelet
count usually returns to baseline levels within 1 week of discontinuing
heparin.
Heparin-induced thrombocytopenia is caused by an IgG that activates
platelets via their Fc II receptors. The major target antigen
is a heparin sulphate/platelet factor IV complex that localizes
the IgG on the platelet surface. The thrombogenic diathesis
results from in vivo platelet activation as well as generation
of procoagulant platelet-derived microparticles. In addition,
heparin-induced thrombocytopenia IgG has been shown to activate
endothelium in vitro via recognition of a heparin sulfate/platelet
factor IV complex.
Laboratory Testing
Platelet activation assays that use washed target platelets
have a sensitivity and specificity for heparin-induced thrombocytopenia
of at least 95%.170 Typically, heparin-induced thrombocytopenia
IgG activates platelets at low (0.5 to 1.0 U/mL) but not high
(10 to 100 U/mL) concentrations of heparin. Aggregation studies
in which citrated plasma is used are much less sensitive to
heparin-induced thrombocytopenia IgG than assays in which washed
platelets are used. An ELISA assay with the platelet factor
IV/heparin target antigen has been developed that shows good
concordance with the platelet activation assay.
Although heparin-induced thrombocytopenia is much less common
with LMWH preparations than standard heparin, in vitro studies
indicate that LMWHs show immune cross-reactivity in ~70% of
instances. In contrast, in vitro cross-reactivity is much less
common (~10%) with the heparinoid Orgaran, which has been used
successfully as a substitute for heparin in patients with heparin-induced
thrombocytopenia.
Treatment
Two different antithrombotic agents have been evaluated in descriptive
studies. These are Orgaran and the defibrinogenating snake venom
ancrod (Arvin). Intravenous administration of Orgaran produces
immediate onset of anticoagulation after bolus administration.
Ancrod has the advantage of exhibiting no cross-reactivity with
heparin, but there is a delay of ~12 hours before effective
defibrinogenation can be achieved. In addition, neither thrombin
generation nor platelet activation are inhibited by ancrod348
and the magnitude of the anticoagulant effect is less predictable
than with Orgaran. Ancrod is also contraindicated in patients
with disseminated intravascular coagulation or septicemia. Long-term
(ie, >3 weeks) anticoagulation with ancrod is limited by
development of antibodies that render patients resistant to
its effects.Unfortunately, neither of these agents is approved
for use in the United States, but they can be obtained for compassionate
use. Initial experience with hirudin from Europe is very promising,
but it is not approved for use in North America.
Complications of Oral Anticoagulants
Bleeding is by far the most common complication of oral anticoagulant
therapy. Randomized studies have shown that the risk of bleeding
is influenced by the intensity of anticoagulation, and several
studies have shown that the risk of clinically important bleeding
is reduced by lowering the therapeutic range for the INR from
3.0 to 4.5 to 2.0 to 3.0. Although this difference in anticoagulant
intensity is produced by a mean reduction in the dose of warfarin
of only ~1 mg, the effect on bleeding is profound. Randomized
studies have also shown that the rate of oral anticoagulant-induced
bleeding is increased by concomitant use of high doses of aspirin
that both impair platelet function and produce gastric erosions.
Multivariate analysis of cohort studies also suggests that risk
of bleeding is influenced by the underlying clinical disorder.
These studies reported that the risk of major bleeding is increased
by age >65 years, a history of stroke or gastrointestinal
bleeding, and the presence of serious comorbid conditions such
as renal insufficiency or anemia. Bleeding that occurs when
the INR is <3.0 is frequently associated with an obvious
underlying cause or an occult gastrointestinal or renal lesion.
Drugs that are known to interact with coumarins should be avoided
if possible.361 However, if concomitant use of drugs that interact
with warfarin is necessary, PT should be monitored more frequently
in the first few days to weeks of combined use to anticipate
a change in dosage. Furthermore, all new drugs should be viewed
as having the potential to interact with coumarins, and the
frequency of PT monitoring should be increased in the initial
period after introduction. Drugs known to inhibit platelet function
should be avoided unless prescribed to augment the antithrombotic
effects of warfarin. For example, low-dose aspirin (100 mg/d)
augments the antithrombotic effects of coumarins in patients
with prosthetic heart valves but at an increased risk of minor
bleeding.
The frequency of bleeding depends very much on intensity of
the anticoagulant effect and patient-related risk factors. If
moderate-dose anticoagulant therapy is used to prolong the INR
to between 2.0 and 3.0, bleeding is relatively uncommon. Most
episodes occur in patients with a potential bleeding source
such as a peptic ulcer, gastritis, renal calculus, or malignancy.
Bleeding complications in patients on long-term anticoagulant
therapy tend to occur early and may unmask an underlying local
source. In randomized trials of moderate-intensity warfarin
(INR, 2.0 to 3.0) in patients with nonvalvular atrial fibrillation
versus untreated control subjects, the typical annual incidence
of major bleeding was between 1.0% and 1.5% in the warfarin
groups and 0.5% to 1.0% in the control groups. However, patients
selected for these trials were at low risk for bleeding, so
in practice, bleeding on warfarin is higher than reported by
these studies.
Management of Bleeding
If bleeding occurs during oral anticoagulant treatment in a
patient with VTE, management depends on severity of bleeding,
INR at the time of bleeding, and whether or not the patient
has completed most of the prescribed course of anticoagulant
therapy.
If the INR is above the therapeutic range, treatment can be
discontinued until bleeding has stopped and then reintroduced
cautiously at a lower intensity. If the INR is within the therapeutic
range, a local source of bleeding should be sought, particularly
if bleeding is gastrointestinal or from the urinary tract. However,
if the INR is markedly prolonged, it is not usually necessary
to look for a source of bleeding.
If bleeding is life-threatening and the INR prolonged, the
coagulation defect should be reversed immediately by infusion
of plasma, and vitamin K1 should be administered in a dose of
10 mg to 25 mg either intravenously by slow infusion or by subcutaneous
injection.
If bleeding is not life-threatening and the INR is markedly
prolonged, then the anticoagulant effect can be reversed by
administering 5 mg vitamin K1 by subcutaneous injection.
Vitamin K1 can interfere with subsequent warfarin therapy when
doses of 10 mg or more are used, and it can cause refractoriness
to further warfarin therapy for up to 2 weeks.
Skin Necrosis
The most important nonhemorrhagic side effect of warfarin is
skin necrosis. This uncommon complication is usually observed
on the third to eighth day of therapy and is caused by extensive
thrombosis of the venules and capillaries within the subcutaneous
fat. An association has been reported between warfarin-induced
skin necrosis and protein C deficiency, and less commonly, protein
S deficiency, but this complication can also occur in persons
without a deficiency. A role for protein C deficiency seems
probable and is supported by the similarity of the lesions to
those seen in neonatal purpura fulminans, which complicates
homozygous protein C deficiency. The reason for the unusual
localization of the lesions to subcutaneous fat deposits remains
a mystery. The optimal technique for initiating anticoagulant
therapy in patients with known protein C or protein S deficiency
is uncertain. A reasonable empirical approach is to start with
an initial course of heparin, begin warfarin at a maintenance
dose of 5 mg, and give both anticoagulants in combination for
~7 days.
In patients who develop warfarin-induced skin necrosis, warfarin
should be discontinued, vitamin K1 should be given to increase
levels of protein C, and full doses of heparin should be administered
to achieve a rapid anticoagulant effect. Treatment of patients
with warfarin-induced skin necrosis who require anticoagulant
therapy for an indefinite period is difficult. These patients
can be treated with subcutaneous heparin long term, but this
is inconvenient and carries a risk of osteoporosis. It might
be safe to reintroduce warfarin in low doses initially in combination
with heparin and to use combined treatment for 10 to 14 days,
during which time the warfarin dose is gradually increased.
It should be noted, however, that heparin may not terminate
coumarin necrosis, and some have reported that heparin failed
to prevent continuing skin necrosis in homozygous protein C
deficiency with very low protein C levels.
Management When Anticoagulants Are Stopped
Management of thromboembolism is influenced by the nature of
the thromboembolic event, the time during the course of anticoagulant
therapy that bleeding occurred, and the INR level during bleeding.
If bleeding occurs in a patient with calf vein thrombosis who
has received an adequate course of heparin therapy, then oral
anticoagulant therapy can be stopped and replaced with low-dose
heparin 5000 U twice daily SC. If bleeding occurs toward the
end of a course of anticoagulant therapy (eg, >2 months after
starting treatment) in a patient with proximal vein thrombosis,
a decision can be made to terminate the course of anticoagulants.
Long-term Warfarin Therapy and Elective Surgery
Long-term anticoagulation is indicated in patients with a sustained
high risk of arterial or venous thromboembolism. Elective surgery
in such patients can be difficult. A number of approaches are
available, but none have been evaluated in appropriately designed
clinical studies. In general, the management choice should take
into account the risk of thromboembolism if the patient is left
untreated and the risk of bleeding if aggressive perioperative-operative
anticoagulation is used.
The least complicated approach is to stop oral anticoagulants
and perform elective surgery when the INR has returned to the
normal range. Oral anticoagulants can then be started postoperatively
in combination with low-dose or full-dose heparin, the choice
of heparin regimens depending on the anticipated risk of postoperative
bleeding. White and associates have reported that it takes ~4
to 5 days for an INR between 2.0 and 3.0 to return to the normal
range after warfarin is discontinued. Stopping anticoagulants
4 to 5 days preoperatively is appropriate in patients with atrial
fibrillation or mechanical prosthetic valves because the risk
of thrombosis in untreated patients is <10% per year. This
annual incidence translates to a risk of thromboembolism of
<0.1% over the 2 or 3 days that patients are without protection.
A modification of this approach, which would further decrease
risk, is to delay stopping warfarin until 2 days before surgery
and reverse the anticoagulant effect with a 1- or 2-mg dose
of vitamin K by subcutaneous injection, repeated if the INR
is still prolonged 24 hours after injection. Low doses of vitamin
K1 (1 to 2 mg) have been reported to lower the INR within 24
hours without producing warfarin resistance when anticoagulant
treatment is reintroduced postoperatively.
A more aggressive approach should be considered for patients
who are at high risk of developing postoperative venous thrombosis.
These include patients with a past history of venous thrombosis
or recurrent venous thrombosis, particularly if they have a
persistent risk factor for venous thrombosis. Two treatment
options are available for these high-risk patients. The first
is to lower the dose of warfarin and perform the operation at
an INR of ~1.5; this approach has been shown to be safe and
effective in preventing postoperative venous thrombosis in high-risk
orthopedic surgical patients. The second option is to stop warfarin
and replace the oral anticoagulant with full-dose heparin by
continuous intravenous infusion preoperatively, stop heparin
6 hours before surgery, and restart anticoagulant therapy with
heparin and warfarin postoperatively. Postoperative heparin
should be delayed for at least 12 hours or longer if there is
evidence of excessive bleeding or risk of serious postoperative
bleeding.
Approach to Thrombophilia
Thrombophilia is defined as an increased tendency to thrombosis.
It can be inherited or acquired. The term implies that there
is an ongoing stimulus to thrombogenesis or a defect of the
natural anticoagulant or fibrinolytic mechanism that predisposes
the patient to thrombosis or recurrent thrombosis. Thrombophilia
is often suspected on clinical grounds because a patient presents
with clinical features listed in Table 10.
Table 10. Clinical Manifestations of Thrombophilia
Family history of venous thrombosis
Thrombosis at a young age
Recurrent venous thrombosis
Idiopathic venous thrombosis
Venous thrombosis following minimal provocation (eg, antepartum
thrombosis, thrombosis after a long car ride or airplane flight
while taking oral contraceptives)
Venous and arterial thrombosis in combination
Thrombosis in an unusual site
Inferior vena cava
Mesenteric vein thrombosis
Cerebral vein thrombosis
Renal vein thrombosis
Hepatic vein thrombosis
Axillary vein thrombosis
Recurrent thrombosis despite adequate anticoagulant therapy
(malignant disease)
--------------------------------------------------------------------------------
Some clinical manifestations of thrombophilia are particularly
characteristic of special thrombophilic disorders. These deficiencies
are shown in parentheses.
The most important of these clinical features are idiopathic
thrombosis, a family history of thrombosis, recurrent thrombosis,
thrombosis at a young age, thrombosis after trivial provocation,
and thrombosis in an unusual site. The spectrum of thrombophilia
can range from an increased tendency to thrombosis, which is
easily controlled by anticoagulant therapy, to progressive and
intractable thrombosis, which is resistant to all forms of therapy.
The former manifestations of thrombophilia are much more common
than the latter, which is sometimes seen in malignant disease.
Patients are considered thrombophilic if they have laboratory
or clinical disorders that are known to be associated with an
increased risk of thrombosis. Thrombophilic conditions can be
inherited or acquired ( Table 11).
Table 11. Causes of Thrombophilia
| Familial Thrombophilic (Inherited) Disorders |
Acquired Thromboembolic Disorders |
| Established association |
Malignancy |
APC resistance
AT-III deficiency
Protein C deficiency (heterozygous autosomal dominant)
Protein C deficiency (homozygous autosomal recessive)
Protein S deficiency
Dysfibrinogenemia*
|
Antiphospholipid antibody
|
| Unestablished association |
Paroxysmal nocturnal hemoglobinuria
|
Plasminogen deficiency
Heparin cofactor II deficiency
Increased histidine-rich glycoprotein
Decreased plasminogen activator activity |
Myeloproliferative syndrome
|
| |
Nephrotic syndrome
|
| |
Estrogen therapy for infertility |
| |
Chemotherapy for cancer
Reduction in postoperative fibrinolytic activity
|
The inherited molecular abnormalities associated with an increased
risk of venous thromboembolism are AT-III deficiency, protein
C and protein S deficiencies, dysfibrinogenemia, and activated
protein C resistance. Of these, AT-III deficiency and protein
C and protein S deficiency are seen in ~10% of patients with
idiopathic venous thrombosis and dysfibrinogenemia in <0.5%.
Thus, until recently only ~10% of patients with clinically suspected
thrombophilia had an associated observable genetic defect. This
state of affairs changed dramatically with the discovery of
activated protein C (APC) resistance by Dahlback and colleagues
in 1993. These investigators found that adding APC to plasma
obtained from a patient with recurrent thrombosis failed to
prolong aPTT, and the term "APC resistance" was introduced
to describe the abnormality. This laboratory finding was confirmed
by other investigators, who reported that between 20% and 60%
of patients with recurrent thrombosis had APC resistance.
APC resistance is transmitted in an autosomal dominant manner.
The genetic defect underlying many cases of APC resistance was
described in 1994. Most patients with APC resistance have a
mutant factor V molecule (factor V Leiden), which resists proteolysis
by APC when activated to factor Va. The genetic defect causing
APC resistance is common; it occurs in ~5% of normal populations.
Sixty percent of women in whom thrombosis occurred while they
were taking oral contraceptives have been reported to have APC
resistance.
Thus, 1 of the 4 inherited disorders (APC resistance, protein
C deficiency, protein S deficiency, and AT-III deficiency) is
found in at least half of all patients with idiopathic venous
thrombosis.
The main acquired factors that predispose a patient to thrombosis
are the presence of an anticardiolipin antibody (lupus anticoagulant),
malignancy, and chemotherapy for cancer. Less common are paroxysmal
nocturnal hemoglobinuria, myeloproliferative disorders, nephrotic
syndrome, and hormonal treatment for infertility. Other inherited
laboratory abnormalities for which an association has not been
proved are plasminogen deficiency, heparin cofactor II deficiency,
increase in histidine-rich acid glycoprotein, and reduced plasminogen
activator activity due to either increased levels of plasminogen
activator inhibitor or reduced levels of TPA.
In addition, preoperative and postoperative reductions in fibrinolytic
activity associated with increased plasminogen activator inhibitor
and reduced activity of plasminogen activator have been shown
to be associated with an increased risk of postoperative thrombosis.
Investigation of Thrombophilia
Laboratory testing for inherited AT-III, protein C or protein
S deficiency, and resistance to activated protein C should be
performed when the patient is not being treated with anticoagulants,
at a time remote from the acute thrombotic event, and after
excluding the various acquired disorders known to perturb the
levels of some of these naturally occurring anticoagulants.
Whenever possible, the diagnosis of inherited deficiency should
be confirmed by family studies. If anticoagulant therapy is
indicated because of the underlying thrombotic process, then
testing for AT-III deficiency can be done while the patient
is being treated with oral anticoagulants (a low result would
be diagnostic, although an AT-III level in the normal range
would not exclude AT-III deficiency). Assays for protein C and
protein S can be performed while the patient is on high-dose
subcutaneous heparin. Testing for APC resistance with coagulation-based
assays during anticoagulant therapy has been difficult in the
past. The problem can be overcome by using genetic testing for
factor V Leiden or a tissue factor-dependent factor V assay
that permits reliable diagnosis of APC-resistant factor Va in
patients receiving anticoagulant therapy.
Before labeling the patient as having a deficiency, it is important
to repeat the test on several occasions, exclude an underlying
acquired abnormality that could produce a falsely low test result,
and, if possible, perform studies in family members to confirm
the inherited nature of the deficiency. If a laboratory marker
of thrombophilia is found, comprehensive family studies should
be performed to determine whether other family members have
the defect, since asymptomatic carriers should be counseled
about the need for prophylaxis when they are exposed to high-risk
situations. In addition, female patients with thrombophilia
or asymptomatic carriers of AT-III, protein C or protein S deficiency,
and those with factor V Leiden require counseling with regard
to future pregnancies, oral contraceptives, and postmenopausal
estrogen replacement therapy.
Many investigations for an acquired thrombophilic state can
be performed at the same time as assays for inherited thrombophilia.
The antiphospholipid antibody syndrome should be investigated
by both an antiphospholipid antibody test and tests for circulating
anticoagulants. Malignancy should be suspected in patients without
other detectable causes for venous thrombosis who present with
idiopathic venous thrombosis, recurrent venous thrombosis, including
recurrent superficial venous thrombosis, and thrombosis in an
unusual site such as the portal vein, mesenteric vein, or vena
cava. Malignancy should also be suspected in patients who develop
recurrent thrombosis despite adequate oral anticoagulant or
heparin therapy and in patients with the syndrome of thrombophlebitis
migrans. Malignancy is usually suspected on the basis of compatible
clinical manifestations, although patients with occult malignancy
can present with thrombosis. However, in patients with first-episode
typical DVT, without special features of a thrombophilic state,
expensive or uncomfortable investigations for malignant disease
should not be performed if simple investigations (complete blood
count, chest radiograph, and fecal occult blood testing) are
negative. If malignancy is suspected, a computed tomography
scan of the abdomen and/or an ultrasound of the abdomen and
pelvis should be performed. If there is evidence of an iron
deficiency anemia or if testing for fecal occult blood is positive,
then endoscopy and/or barium studies should be performed to
investigate the lower and upper gastrointestinal tract.
Screening for a myeloproliferative disorder is performed by
a complete blood count, including a platelet count. Paroxysmal
nocturnal hemoglobinuria is suspected from the results of a
complete blood count that usually show anemia with evidence
of either hemolysis or bone marrow hypoplasia and the diagnosis
is confirmed by demonstrating hemoglobinemia, hemoglobinuria,
and hemosiderinuria and by performing Ham's test or Hartman's
sugar/water test. The nephrotic syndrome is suspected if there
is generalized edema, hypoalbuminemia, and proteinuria.
Antiphospholipid Syndrome
Antiphospholipid syndrome can present in a number of ways. Acquired
circulating anticoagulants were first identified in patients
with systemic lupus erythematosus in 1948, and an association
with thrombosis was noted 15 years later. The phenomenon was
called the lupus anticoagulant, but this anticoagulant is not
restricted to patients with systemic lupus erythematosus.
Antiphospholipid syndrome is diagnosed when patients with a
positive assay for antibody against phospholipids have one or
more of the following manifestations: venous or arterial thrombosis,
thrombocytopenia, or recurrent fetal wastage. The laboratory
tests developed to detect lupus anticoagulants include aPTT,
kaolin clotting time, dilute phospholipid test, platelet neutralization
tests, and tissue thromboplastin inhibition tests; the coexistence
of antibodies to cardiolipin has been noted. IgG and IgM antibodies
to phosphatidylserine, phosphatidylinositol, phosphatidic acid,
and cardiolipin were found in subjects with the lupus anticoagulant.
Both arterial and venous thromboses occur in patients with
antiphospholipid syndrome. Thrombosis in unusual sites has been
described, including the Budd-Chiari syndrome, portal vein thrombosis,
and inferior vena caval thrombosis. Antiphospholipid syndrome
has been reported in patients with transient ischemic attacks,
multi-infarct dementia, and myocardial infarction410; thrombosis
of the cerebral, splenic, portal, hepatic, renal, subclavian,
and retinal veins and of the inferior vena cava; and coumarin
skin necrosis, adrenal gland hemorrhage, and infarction. Antiphospholipid
syndrome may be the cause of cerebral ischemic events in patients.
Myeloproliferative Syndromes
Myeloproliferative syndromes have been associated with cerebral
or mesenteric venous thrombosis; thrombosis of the splenic vein,
portal vein, and hepatic vein; and a variety of arterial events,
including strokes, myocardial infarction, vague neurological
symptoms, and ischemia of the fingers and toes.434-442 In polycythemia
vera, hypercoagulability is caused by increased viscosity of
blood resulting from the increased hematocrit. Treatment with
phlebotomy should be aimed at maintaining the hematocrit in
the low-normal range (40% to 45%). However, other agents may
be needed to lower the platelet count.
Anagrelide, a quinazolin compound, is a new drug available
for the management of thrombocythemia. Silverstein et al444
have accumulated data on more than 500 patients with essential
thrombocythemia treated with anagrelide, with a 93% success
rate in reducing platelet count to less than 650×109/L.
This reduction is associated with parallel relief of symptoms
(transient ishemic attacks, venous thrombosis, erythromelalgia)
without a significant number of side effects. Fluid retention
may be a side effect of its use in some patients, including
those with congestive heart failure. Anagrelide may become front-line
therapy for thrombocythemic states not only because of its selective
efficacy in inhibiting megakaryocyte maturation but because
no documented leukemogenic effect has been found after 8 years
of use.
Anagrelide will not likely displace two other agents, hydroxyurea
and -interferon, that are effective in treatment of thrombocythemia.
Even though rare cases of leukemia have followed the use of
hydroxyurea in myeloproliferative disorders, the ease and effectiveness
of its low-cost administration continue to make it a valuable
agent for these patients. The cytokine -interferon requires
frequent subcutaneous injection of an expensive agent whose
use may be accompanied by significant side effects. Because
the side effects of interferon are usually manageable and transitory,
and because injections may be decreased from three times weekly
to once weekly, this cytokine should be considered for some
patients.
No absolute relation between platelet number and frequency
of thrombotic complications has been established in thrombocythemia,
but it has been demonstrated that the longer the platelet count
is less than 600×109/L, the lower the incidence of thrombotic
complications. High vascular complication rates in patients
>60 years and in patients with a history of a thrombotic
event make both of these patient groups candidates for therapeutic
intervention. A randomized trial of hydroxyurea has demonstrated
a significant reduction in thrombotic events (3.6% versus 24%)
in favor of those treated with drug therapy. In younger patients
with asymptomatic thrombocythemia, therapeutic recommendations
should remain conservative.
Trousseau's Syndrome
Most patients with malignancy and thrombosis do not have Trousseau's
syndrome, which refers to a thrombus that is characteristically
migratory and recurrent. Thrombosis occurs in leg veins, neck
veins, superficial veins of the thorax and abdomen, the dorsal
vein of the penis, subclavian and axillary veins, cerebral veins,
and visceral veins. Arterial thromboses can also occur. Thrombosis
tends to be associated with mucinogenic adenocarcinoma.
Treatment of Trousseau's syndrome can be difficult. Coumarin
is usually not effective. Heparin often controls the thromboembolic
manifestations and can be given long term on an outpatient basis
in full therapeutic doses.
Management of Thrombophilia
All thrombophilic patients should receive prophylaxis in high-risk
situations, and some require long-term anticoagulant therapy.
Lifelong anticoagulant treatment should be considered for thrombophilic
patients with a documented episode of thrombosis, with or without
a laboratory abnormality, while thrombophilic patients without
documented evidence of thrombosis should receive prophylaxis
when exposed to high-risk situations (eg, surgery, prolonged
immobilization, pregnancy). In patients with polycythemia vera,
the hematocrit and platelet count should be controlled with
appropriate therapy. In addition, female patients with thrombophilia
and asymptomatic carriers of AT-III, protein C or protein S
deficiency, and the factor V gene mutation require counseling
about future pregnancy, use of oral contraceptives, and postmenopausal
estrogen replacement therapy.
Descriptive studies in AT-III deficiency suggest that risk
of thrombosis in the unprotected pregnant patient is very high.
While the risk of thrombosis for protein C and protein S deficiencies
during pregnancy is lower than for AT-III deficiency, risk of
postpartum thrombosis is high in all three groups.
Three approaches can be used to treat a thrombophilic patient
during pregnancy:
Full-dose heparin by subcutaneous injection every 12 hours
for the duration of pregnancy. The dose should be adjusted to
maintain the aPTT in a range equivalent to a heparin level of
0.2 to 0.4 U/mL by protamine titration.
Low-dose heparin 5000 U SC every 12 hours for the duration of
pregnancy.
Frequent surveillance with IPG or duplex ultrasound imaging
for the duration of pregnancy.
The first option should be considered for patients who must
take oral anticoagulants as long as they live, including those
at risk of cardiac embolism; patients with previous venous thrombosis
associated with deficiencies of AT-III, protein C, and protein
S or with lupus anticoagulant; and any patient with two or more
previous episodes of venous thrombosis. The second option should
be considered in asymptomatic carriers of AT-III deficiency
and patients with previous idiopathic venous thrombosis or thrombosis
during an uncomplicated pregnancy. It would be reasonable to
consider either the second or third option in asymptomatic carriers
of protein C or protein S deficiency and the third option in
patients with only one episode of previous venous thrombosis
after provocation.
Jack Hirsh, MD; John Hoak, MD:Management of
Deep Vein Thrombosis and Pulmonary Embolism:Circulation. 1996;93:2212-2245
DIAGPEXray-fig.8(James T.T.Chen,Hurst's The Heart,10th Edition,Chapter12,page320;DIAGPEANGIOG-fig.9(Lewis
J.Rubin,Pulmonary Hypertension,Hurst's The Heart,10th Edition,Chapter
52 ,page 1613);DIAGPEPERFScan-fig10,diagpeperfscan-fig11(Sheldon
Baum and others,Atlas of Nuclear Medicine Imaging,Chapter2,pages
87,91)
DiagPEXray-fig.8'',DiagPEECG-fig.8''' ,DiagPE ECG-fig.8''''
(Paul Wood,Diseases of the Heart and Circulation,Pages 815,812,813).
legveinTE-fig.1b,legDVT-fig.1c',venogram-fig.-1:Deep
venous thrombosis,Provided by A.D.A.M.
http://health.yahoo.com/health/encyclopedia/000156/tn.html