PERIPHERAL VASCULAR DISEASE
(PERIPHERAL ARTERIAL DISEASE; PAD; ARTERIOSCLEROSIS OBLITERANS)
Arteriosclerosis of the extremities
is a disease of the blood vessels characterized by narrowing
and hardening of the arteries (see Fig.70 under atherosclerosis
of coronary arteries ) that supply the legs and feet (Fig.1: leg
arteries).
This causes a decrease in blood flow that can injure nerves
and other tissues.
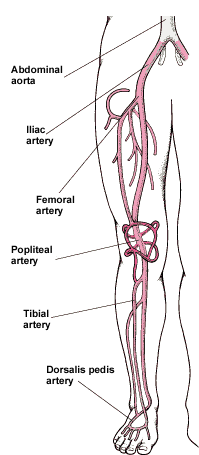
Fig.1: Leg arteries
Causes, incidence, and risk factors
Arteriosclerosis, or "hardening
of the arteries," commonly shows its effects first in
the legs and feet. The narrowing of the arteries may progress
to
total closure (occlusion) of the vessel. The vessel walls become
less elastic and cannot dilate to allow greater blood flow
when
needed (such as during exercise). Calcium deposits in the walls
of the arteries contribute to the narrowing and stiffness.
The
effects of these deposits may be seen on ordinary X-rays and
on angiograms (see Fig.53 under coronary
atherosclerosis).
This is a common disorder, usually
affecting men over 50 years old. People are at higher risk
if
they have a personal or family history of coronary artery disease
(heart disease) or cerebrovascular disease (stroke) (see Fig4:Cerebrovascpad
below), diabetes, smoking, hypertension, or kidney disease
involving hemodialysis.
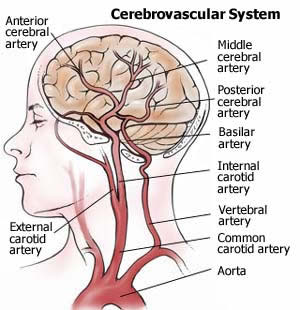
Fig4: Cerebrovascpad Cerebrovascular
disease is atherosclerosis that occurs in the blood vessels
supplying blood to the brain. Most cerebrovascular disease
occurs within arteries not in the brain itself, but in the
neck (carotid
arteries), supplying oxygen-rich blood to the brain. Atherosclerosis
occurring within these neck arteries is a form of peripheral
arterial disease (PAD) called carotid artery disease. Carotid
artery disease accounts for well over 95 percent of symptom
causing cerebrovascular disease.
Symptoms
Often, symptoms affect one limb.
If arteriosclerosis exists in both limbs, the intensity is usually
different in each.
Leg pain (intermittent claudication) occurs with exercise (such
as walking),relieved with rest.
Numbness of the legs or feet at rest
Cold legs or feet
Muscle pain in the thighs, calves, or feet
Loss of hair on the legs and/or feet
Change of color of the legs
Paleness or blueness (cyanosis)
Pulse, weak or absent in the limb
Walking/gait abnormalities
Signs and tests
An examination may show arterial
bruits (whooshing sound heard with the stethoscope over the
artery), decreased or absent pulse in the extremities, or decreased
blood pressure in the affected limb.
Blood tests may show high cholesterol.
Peripheral artery disease may
be revealed by:
An abnormal ratio between the
blood pressure of the ankle and arm (ankle/brachial index,
or ABI).
A doctor suspects an obstruction
based on the symptoms the patient describes and a pulse that's
diminished or absent below a certain point in the leg. Doctors
estimate blood flow to a person's legs in several ways, including
comparing blood pressure at the ankle with blood pressure in
the arm. Normally, the ankle pressure is at least 90 percent
of the arm pressure, but with severe narrowing it may be less
than 50 percent.
The first and most important
noninvasive test for PAD is the ankle-brachial index (ABI).
This test may be performed in the physician's office and has
only 4 requirements:
1) basic understanding of how
to perform an ABI.
2) basic knowledge of arterial anatomy.
3) handheld continuous-wave Doppler ultrasonic probe and acoustic
gel.
4) sphygmomanometer.
The ABI compares the blood pressure
obtained with the handheld Doppler in the dorsalis pedis or
posterior tibial artery (whichever is higher) with the blood
pressure in the higher of the 2 brachial pressures.
In general, an ABI of >/=0.90
is considered normal, >0.40 to <0.90 reflects mild to
moderate PAD, and </=0.40 suggests severe arterial occlusive
disease.
The ABI has emerged as one of
the most potent markers of diffuse atherosclerosis, CV risk,
and overall survival in various patient populations; an abnormal
ABI indicates a 3-fold CV risk. In a study of 2023 middle-aged
men screened with ABI,[4] the relative risks for mortality
from all causes, CV causes, and coronary causes were significantly
higher among patients with an ABI of >/=0.90 than among
patients with a normal ABI. Similarly, in a study of 1492
women >65
years of age,[5] the relative risks for all-cause mortality,
heart disease, and CV disease were significantly greater when
the baseline ABI was </=0.90. In a study of >5000 men
and women >/=65 years of age,[6] results showed that the
lower the ABI, the greater the incidence of CV risk factors
and clinical CV disease.
Limitations of the ABI include:
1) A normal ABI in the face
of abnormal peripheral arterial circulation (ie, a false-negative
result). In elderly patients or patients with end-stage renal
disease or, more commonly, diabetes mellitus, the ankle arteries
may have calcification in the medial layer. Therefore, when
the physician compresses the sphygmomanometer and listens
with
the Doppler probe, the Doppler signal does not disappear at
a pressure of >/=250 mm Hg. This reading does not translate
into a normal ABI but instead indicates vessel calcification,
and more sophisticated noninvasive tests are required.
2) A normal ABI in patients
with classic symptoms suggesting intermittent claudication
and PAD.
Patients with moderate disease of the infrarenal aorta or iliac
arteries may have normal arterial circulation at rest but
when
exercised demonstrate a decrease in ankle pressure. Therefore,
a resting study is inadequate for patients with exertional
symptoms
of intermittent claudication. In this situation, an exercise
arterial study should be performed to determine the true etiology
of exertional limb pain.
The diagnosis may be confirmed
by certain tests:
Segmental Limb Pressures and
Pulse-Volume Recordings
Once the ABI has been performed,
which provides objective evidence of the presence and overall
severity of PAD in a limb, more
specific information can be obtained in the vascular laboratory.
In the laboratory, segmental limb pressure measurement can
aid
in localizing stenoses or occlusions. Limb pressure cuffs are
placed on the thigh (some laboratories prefer high- and low-thigh
cuffs), calf, ankle, transmetatarsal region of the foot, and
digit. The ABI is calculated and then the pressure is inflated
sequentially in each cuff to ~20 to 30 mm Hg above systolic
pressure. With a continuous-wave Doppler probe placed at a
pedal
vessel, the pressure in the cuff is released gradually, and
the pressure at each segment is measured. A decrease in pressure
between 2 consecutive levels of >30 mm Hg suggests arterial
occlusive disease of the artery proximal to the cuff. In comparing
the 2 limbs, a 20- to 30-mm Hg discrepancy from one limb to
the other at the same cuff level also suggests a significant
arterial stenosis or occlusion proximal to the cuff.
Pulse-volume recordings (PVRs) are plethysmographic tracings
that detect changes in the volume of blood flowing through a
limb. Using equipment similar to the segmental limb pressure
technique, pressure cuffs are inflated to 65 mm Hg, and a plethysmographic
tracing is recorded at various levels. A normal PVR is similar
to a normal arterial pulse wave tracing and consists of a rapid
systolic upstroke and a rapid downstroke with a prominent dicrotic
notch. With increasing severity of PAD, the waveforms become
more attenuated with a wide downslope and, ultimately, virtually
absent waveforms (peripheralart- Figure5).
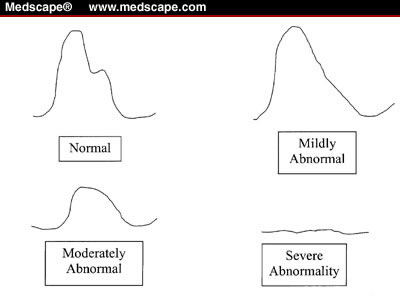
Figure5: Peripheralart Pulse-volume
recording waveforms.
The ABI, segmental limb pressure
measurement, and PVRs (periheral art-Figures 6 and 7) are useful
noninvasive tests for evaluating patients with suspected PAD
or limb discomfort without an obvious cause. These tests are
inexpensive, painless, and reproducible, and the equipment required
to perform them is significantly less expensive than modern
color-flow duplex ultrasonography.
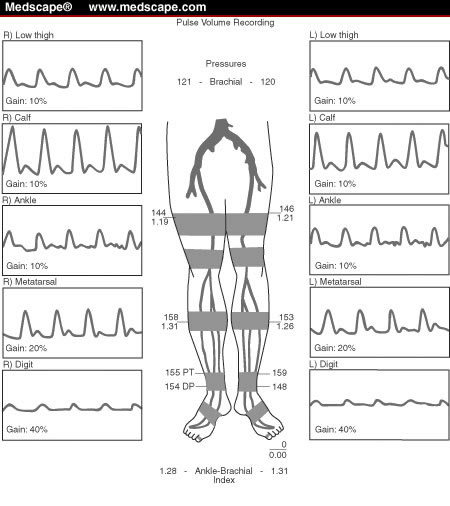
Figure 6: A normal arterial
study with the patient at rest. Note the right ankle-brachial
index
is 1.28 and the left 1.31. The segmental limb pressures and
pulse-volume recordings are normal in both limbs
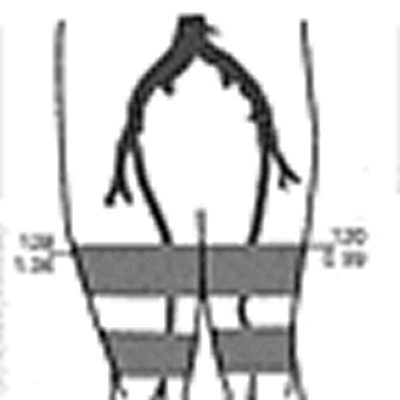
Figure 7: An abnormal arterial
study. The right ankle-brachial index is 0.73 and the left 0.65.
The segmental limb pressures and pulse-volume recordings suggest
evidence of superficial femoral and/or popliteal artery disease
in both lower limbs at rest.
Graded-Exercise Treadmill Test
Segmental limb pressure measurement and PVRs are often combined
with a graded exercise treadmill test. Once the resting pressures
and PVRs are obtained, the patient is asked to walk on a treadmill
at a constant speed, either at a constant grade (2 mph, 12%
incline) or with a variable incline (0% at start, increased
by 3.5% every 2 to 3 minutes). The former method exercises patients
to a maximum of 5 minutes, whereas the latter continues for
a maximum of an 18% incline. The treadmill test:
1) Confirms the diagnosis of
intermittent claudication and PAD.
2) Demonstrates objective functional
limitation of PAD.
3) Documents the effect of therapy
on initial and absolute claudicating distances.
4) Uncovers previously unrecognized
coronary artery disease.
Doppler ultrasound examination of an extremity
With Doppler ultrasound, a probe
is placed on the person's skin over the obstruction, and the
sound of the blood flow indicates the degree of obstruction.
A more sophisticated ultrasound technique, color Doppler produces
a picture of the artery that shows different flow rates in different
colors. Because it doesn't require an injection, it's used instead
of angiography whenever possible.
Native vessel arterial duplex
ultrasonography is generally accepted as a precise method for
defining arterial stenoses or occlusions. The sensitivities
of duplex ultrasonography in detecting occlusions and stenoses
have been reported to be 95% and 92%, respectively, with specificities
of 99% and 97%, respectively.[9] Limitations include tandem
(sequential) stenoses, tibial vessel imaging, and difficulty
in imaging the inflow arteries.
Imaging of the supra- and infrainguinal arteries can be performed
with a 5.0- to 7.5-MHz transducer. The vessels are studied in
the sagittal plane, and Doppler velocities are obtained using
a 60° Doppler angle. Vessels are classified into 1 of 5
categories: normal, 1% to 19% stenosis, 20% to 49% stenosis,
50% to 99% stenosis, and occlusion. The categories are determined
by alterations in the Doppler waveform as well as increasing
peak systolic velocities (PSVs). For a stenosis to be classified
as 50% to 99%, for example, the PSV must increase by 100% compared
with the normal segment of artery proximal to the stenosis.
In lower extremity arteries undergoing duplex ultrasonography,
systolic velocity ratios generally predict degrees of stenosis
(Table). An increase in PSV occurs as a result of increased
flow across an arterial stenosis.
Table. Interpretation of Arterial
Duplex Ultrasonography
| PSV* |
Stenosis Severity |
| Triphasic <100 cm/s |
Normal |
| >30% increase in PSV |
20% to 49% |
| Doubling of PSV( greater than 100% relative to the adjacent proximal segment and reduced systolic velocity distal to the stenosis) |
50% to 99% |
| No Doppler flow in artery |
Occluded |
*Compared with normal arterial
segment proximal to stenosis. PSV = peak systolic velocity.
The same principle is illustrated below in Table 1 from (Stroke. 2007;38:2887.)
©
2007 American Heart Association, Inc. , Progression of Carotid Stenosis Detected by Duplex Ultrasonography Predicts Adverse Outcomes in Cardiovascular High-Risk Patients by
Schila Sabeti, MD ; Oliver Schlager, MD ; Markus Exner, MD ; Wolfgang Mlekusch, MD ; Jasmin Amighi, MD ; Petra Dick, MD ; Gerald Maurer, MD ; Kurt Huber, MD ; Renate Koppensteiner, MD ; Oswald Wagner, MD ; Erich Minar, MD Martin Schillinger, MD
Table 1. Criteria for Quantification of the Degree of Carotid Stenosis by Duplex Ultrasound
| |
0% to 29% |
30% to 49% |
50% to 69% |
70% to 89% |
90% to 99% |
100% |
| PSVICA/PSVCCA |
<1.4 >4.0 |
1.5 to 1.9 |
2.0 to 3.9 |
>4.0 |
Trickle flow |
No flow |
| PSVICA |
<120 |
120 to 149 |
150 to 249 |
>250 |
| PSV indicates peak systolic velocity; ICA, internal carotid artery; and CCA, common carotid artery. Flow velocities are given in cm/s. |
Classification of ICA Stenosis
| Class |
Diameter
Stenosis
(Category) |
Peak
Systolic
Velocity
cm/sec |
Peak
Diastolic
Velocity
cm/sec
|
Systolic
Velocity
Ratio
VICA/VCCA |
Flow Characteristics |
| A |
0% (Nl) |
<125 cm/sec |
<45 cm/sec |
<1.8 |
Minimal or no spectral broadening during the decaleration phase of systole.
Boundary layer separation within the carotid bulb is usually present. |
| B |
1-15%
(mild) |
<125 cm/sec |
<45 cm/sec |
<1.8 |
Minimal spectral broadening during the deceleration phase of systole. |
| C |
16-49%
(mild to moderate) |
<125 cm/sec |
<45 cm/sec |
<1.8 |
Increased spectral broadening during systole until the whole systolic window is filled. |
| D |
50-79% |
>125-325 cm/sec |
>45 cm/sec |
1.8-4.0 |
Marked spectral broadening with a doubling of the systolic velocity with post-stenotic flow ( turbulence and systolic reversal of flow). |
| Medicare |
70-89% |
>325 cm/sec |
|
>4.0 |
91% sensitivity and 87% specifity. NASCET study and Medicare criteria. |
| D+ |
80-99% |
>125 cm/sec |
>140 cm/sec |
>4.0 |
Marked spectral broadening. |
| E |
Total
occlusion |
N/A |
N/A |
N/A |
No flow signal in an adequately visualized ICA (especially distal) with characteristic low or reversed diastolic component in the CCA. A characteristic "thump" may be noted at the stump, or origin of the occlusion. |
Arterial duplex ultrasonography
has been used to guide the percutaneous interventionist toward
the appropriate access site to facilitate endovascular therapy.
This technology also has been used after endovascular therapy
to determine the technical success and durability of the procedure
(peripheralart-Figure 8). However, data indicate that duplex
ultrasonography performed too soon after balloon angioplasty
may overestimate residual stenosis, which may limit the value
of this technology.
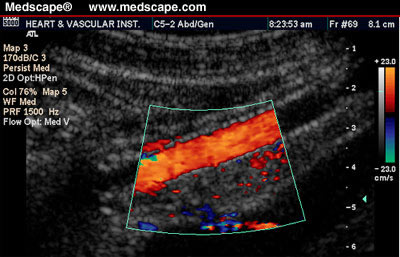
Figure 8: peripheralart Duplex
sonogram of left external iliac artery stent.
Graft Surveillance
In many patients who have undergone surgical bypass graft revascularization,
particularly with saphenous vein, stenoses will develop, which
increase the risk for graft failure. For grafts that have formed
a thrombosis, secondary patency rates are dismal. If the stenosis
is detected and repaired prior to graft thrombosis, an estimated
80% of grafts will be salvaged. Therefore, a well-organized
graft surveillance program is crucial in preserving patency
of the bypass graft. In 1 series of 170 saphenous vein bypass
grafts, 110 stenoses were detected over a 39-month period. Among
grafts that underwent surgical revision once a stenosis was
detected, the 4-year patency rate was 88%, whereas among grafts
that did not undergo revision despite the detection of a stenosis,
the 4-year patency rate was 57%. The use of an intensive surveillance
program has been less beneficial for patients receiving prosthetic
grafts.
The procedure for graft surveillance is similar to native vessel
arterial duplex ultrasonography. The inflow artery to the bypass
graft is initially imaged using a 5.0- to 7.5-MHz transducer
and a Doppler angle of 60°. Then, the proximal anastomosis;
proximal, mid-, and distal graft; distal anastomosis; and outflow
artery are interrogated. The PSV and end-diastolic velocity
are obtained at each segment and compared with those of the
segment of graft proximal to the area being studied. If the
ratio of the PSV within a stenotic segment relative to the normal
segment proximal to the stenosis is >2, this suggests a 50%
to 75% diameter reduction. The addition of end-diastolic velocities
of >100 cm/s suggests >75% stenosis.
Vein bypass grafts should be
studied within 7 days of formation and again in 1 month, and
surveillance should be repeated at 3-month intervals during
the first year. If the graft remains normal after the first
year, follow-up surveillance should be done every 6 months thereafter.
Ankle pressures and waveforms should be obtained at the time
of each surveillance study. The development of a stenosis during
a surveillance examination should prompt consideration of arteriography,
either with contrast or magnetic resonance technology.
Algorithms For Pad Testing
Although vascular laboratories use different algorithms for
diagnosing PAD, some consistent patterns have emerged. ABIs
should be performed on every patient suspected of having PAD.
If specific information regarding the precise location of arterial
stenoses or occlusions is required, complete duplex ultrasonography
should be performed from the infrarenal abdominal aorta through
the tibial arteries. The limitations of this approach include
prolonged examination time, additional cost, and difficulty
in interpreting sequential or tandem lesion severity distal
to a significant proximal stenosis.
If general information regarding the location of arterial occlusive
disease is required, physiologic testing with segmental limb
pressures and PVRs is sufficient; however, these studies must
be performed with the patient both at rest and with exercise.
The exercise portion of the examination confirms whether the
limb pain is due to vascular or nonvascular ("pseudoclaudication")
causes. Pseudoclaudication is usually neurogenic, with a number
of possible causes from lumbar canal stenosis to degenerative
disk disease of the lumbosacral spine. It may be difficult for
the physician to distinguish between symptoms of claudication
and pseudoclaudication based on the history and physical examination
alone. Specific testing will therefore aid in the diagnosis.
Clinical differences in PAD include variable onset of limb pain
with exercise, symptoms at rest and with exertion, and the need
to sit for relief of pain. Exercise testing also provides objective
documentation of the true functional limitation of PAD and can
be used to demonstrate physiologic improvement after intervention.
Duplex ultrasonography is helpful
in identifying areas of vascular trauma, specifically iatrogenic
ones. Pseudoaneurysms occur in </=7.5% of femoral artery
catheterizations and can result in significant complications,
including distal embolization into the native arterial system,
expansion, extrinsic compression on neurovascular structures,
rupture, and hemorrhage. Duplex ultrasonography can identify
these lesions rapidly and accurately, and the use of direct
ultrasound-guided compression or ultrasound-guided thrombin
injection can repair the lesions without the need for invasive
surgical procedures.
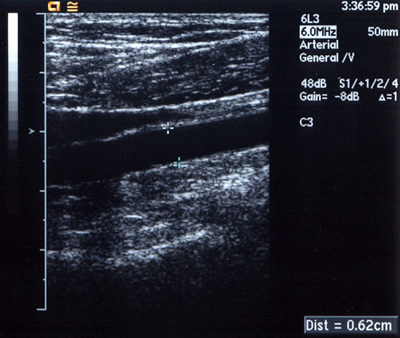
Peripheralart-fig10. Lower-extremity
atherosclerotic arterial disease. Gray-scale sonogram demonstrates
the popliteal artery, which is located between the calipers.
It measures 0.62 cm in diameter. Findings are normal in this
study.
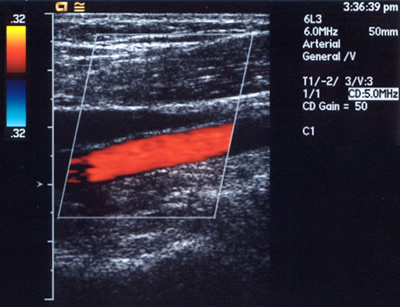
Peripheralart-fig11:Lower-extremity
atherosclerotic arterial disease. Color Doppler sonogram of
the popliteal artery (same patient as in Image peripheralart-fig10).
The red color represents arterial blood flow, its direction,
and its velocity inside the artery. These data were obtained
by measuring the Doppler shifts originating from the sampled
volume inside the artery). Findings are normal in this study.

Movie:
Peripheralart-fig12:
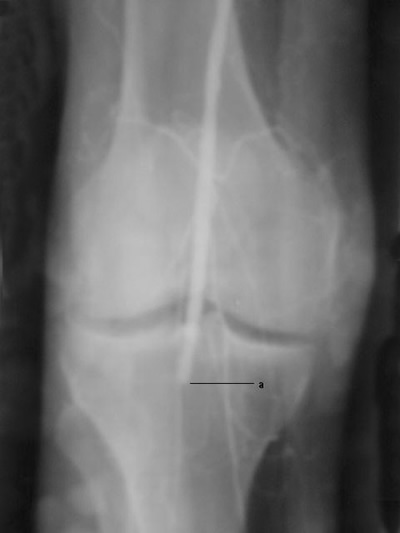
Lower-extremity atherosclerotic arterial disease. Digital
subtraction angiogram
(DSA) illustrates a high-grade short-segment stenosis of the
lumen of the right superficial femoral artery .
Peripheralart-fig13:Lower-extremity
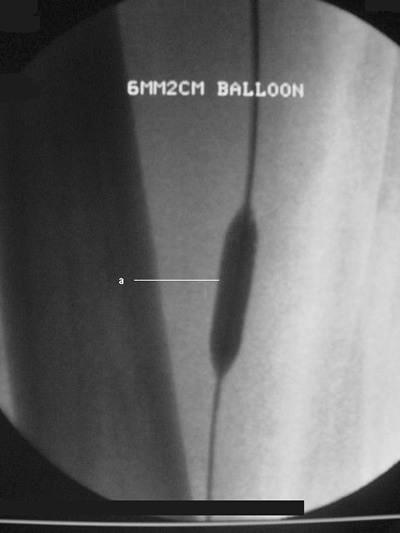
atherosclerotic arterial disease. Conventional catheter angiogram.
The inflated angioplasty balloon technique was performed to
treat the stenosis in the lumen of the right superficial femoral
artery (same patient as in Peripheralart-fig12).
Peripheralartfig14:
Lower-extremity atherosclerotic arterial disease. Cut-film
angiogram illustrates
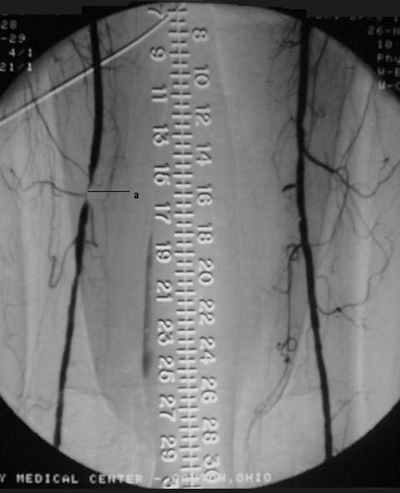
complete embolic occlusion after angioplasty (a). The occlusion
is seen distally at the level of the popliteal artery. The
patient
was treated with percutaneous catheter suction embolectomy.
(Thrombolytic agents such as reteplase or alteplase may also
be used.)
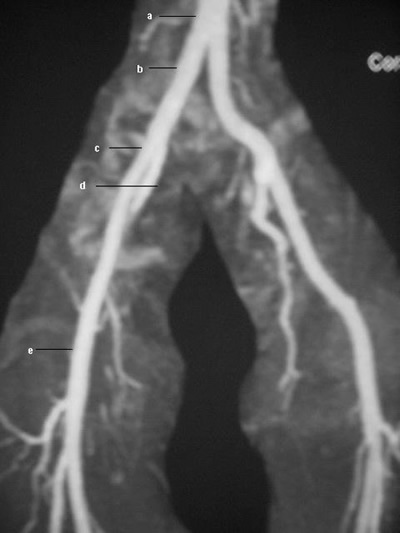
Peripheralartfig15 Lower-extremity
atherosclerotic arterial disease. Magnetic resonance angiogram
(MRA) obtained by using the bolus-chase technique shows the
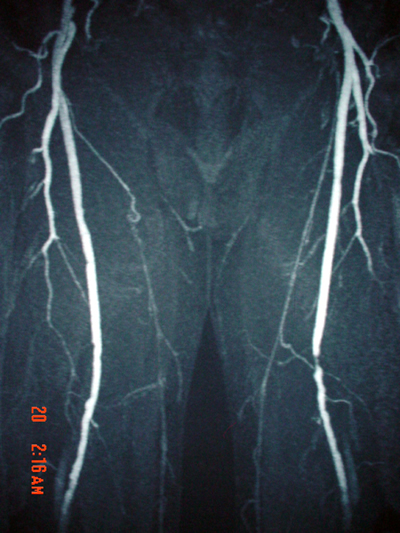
normal anatomy of the lower extremity arterial vasculature,
including the aorta (a), the common iliac artery (b), the external
iliac artery (c), the internal iliac artery (d), and the common
femoral artery (e).
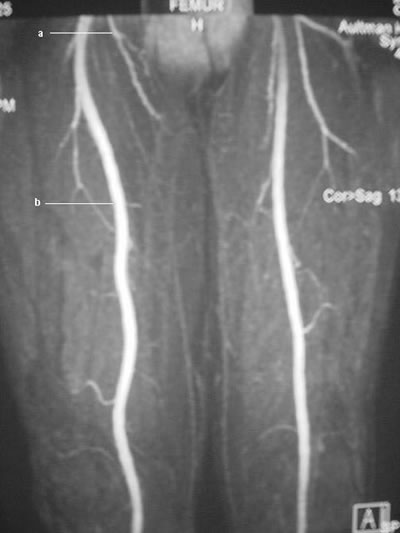
Peripheralart-fig16:Lower-extremity
atherosclerotic arterial disease. Magnetic resonance angiogram
(MRA) obtained by using the bolus-chase technique shows the
normal anatomy of the lower-extremity arterial vasculature,
including the deep femoral artery (a) and the superficial femoral
artery (b).
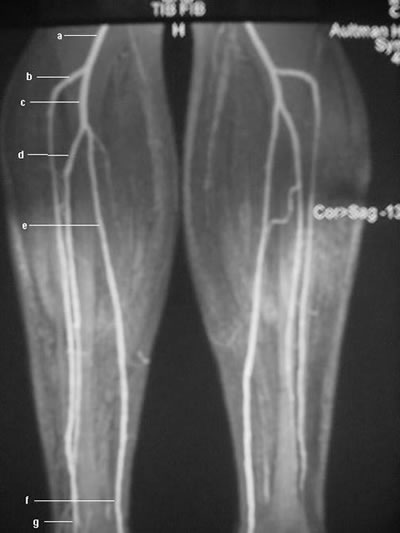
Peripheralart-fig17.Lower-extremity
atherosclerotic arterial disease. Magnetic resonance angiogram
(MRA) obtained by using the bolus-chase technique shows the
normal anatomy of the lower-extremity arterial vasculature,
including the popliteal artery (a), the anterior tibial artery
(b), the tibioperoneal trunk (c), the peroneal artery (d), and
the posterior tibial artery (e).
Peripheralart-figPeripheralart-fig18:Lower-extremity
atherosclerotic arterial disease. This magnetic resonance angiogram
(MRA) of the lower extremities was obtained by using the bolus-chase
technique. A short-segment high-grade stenosis is present in
the middle of the left superficial femoral artery. Note the
collateral arterial supply.
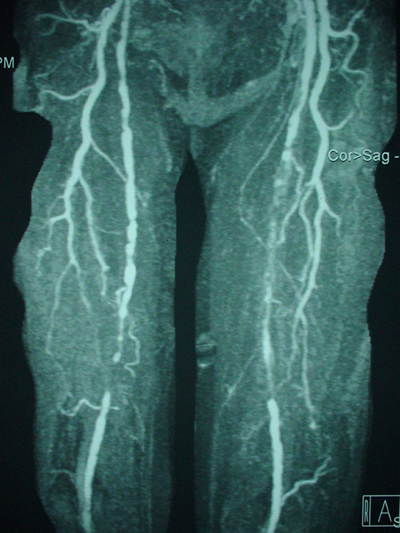
Peripheralart-fig19:Lower-extremity
atherosclerotic arterial disease. This magnetic resonance angiogram
(MRA) of the lower extremities was obtained by using the bolus-chase
technique. Atherosclerotic disease involves the bilateral superficial
femoral arteries. Note the multiple lesions, which are primarily
in the middle portions, and the large collateral arterial supply.
Lower-extremity atherosclerotic arterial disease. This magnetic
resonance angiogram (MRA) of the lower extremities was obtained
by using the bolus-chase technique. Atherosclerotic disease
involves the bilateral superficial femoral arteries. Note the
multiple lesions, which are primarily in the middle portions,
and the large collateral arterial supply.
Angiography of the arteries
in the legs (arteriography) (see peripheralart-fig 9,12,13,14
above;).
Arteriography remains the most
accurate and informative test. Arteriography is the criterion
standard, but it is considered an invasive diagnostic method.
This examination is associated with complications such as hematoma
at the puncture site, those due to radiation exposure, intimal
flap dissection, or arterial wall rupture, and nephrotoxicity
due to the intravenous contrast material (which poses greater
risk because of the common association of Lower Extremity Peripheral
Arterial Disease with renal arterial disease and renal disease).
Therefore, arteriography is preserved for preoperative evaluation
only.
In angiography, a solution
that's opaque to x-rays is injected into the artery. Then
x-rays are
taken to show the rate of blood flow, the diameter of the artery,
and any obstruction.Angiography may be followed by angioplasty
to open up the artery (Peripheralart-fig12; Peripheralart-fig13;
Peripheralart-fig22).
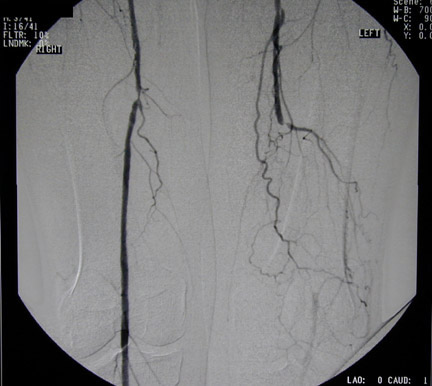
Peripheralart-fig9:This angiogram
shows a superficial femoral artery occlusion on one side (with
reconstitution of the suprageniculate popliteal artery) and
superficial femoral artery stenosis on the other side. This
is the most common area for peripheral vascular disease.
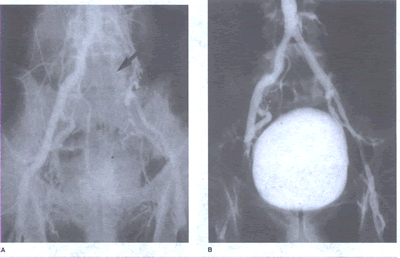
Peripheralart-fig22:A. Aortogram
with pelvic runoff views of a 61-year-old man arterywith left
thigh and buttock claudication and common iliac artery occlusion
(arrow).B.After recannalization of the totally occluded left
common iliac artery(arrow),normal flow was reestablished to
the left leg with percutaneous balloon angioplasty and the placement
of an iliac stent.
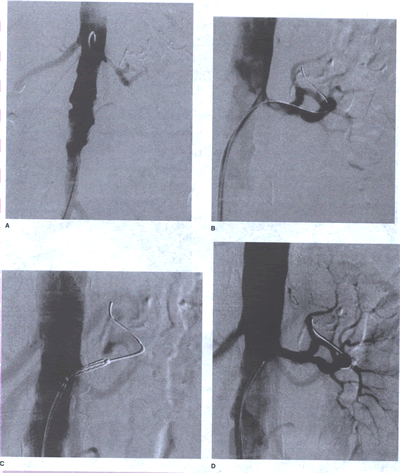
Peripheralart-FIGURE 23: Aortogram
demonstrating an isolated left renal artery stenosis in a 71-year-old
woman with poorly controlled hypertension and normal renal function.
B. Balloon angioplasty produced local dissection with residual
stenosis. C and D. After implantation of an AVE stent, full
resolution of the hemodynamically significant lesion was achieved.
The patient continues to require two medications to control
her hypertension.
Intravascular ultrasound (IVUS)
of the extremity
An MRI scan
Magnetic resonance angiography
(MRA) (see peripheralart-fig.15,16,17,18,19 below) is a test
that produces images of arteries and veins with similar accuracy
to invasive angiograms but without puncturing the artery to
inject dye. MRA is also used to identify small arteries in
the lower leg that cannot be seen or detected with angiograms
or
other testing methods.
MRA is noninvasive and does
not require the use of ionizing radiation, and the contrast
agent used is relatively non-nephrotoxic. This modality is associated
with limitations such as its cost, its availability, the limited
depiction of small vessels, its contraindications, and the possible
overestimation of the degree of stenosis.
Recently, MRA has emerged as
a safe and noninvasive alternative to conventional angiography
in the diagnosis of lower-extremity vascular disease. Using
MRA studies, a radiologist should be able to detect signs of
narrowing (stenosis), dilatation (aneurysm) in the vessel, or
a complete interruption of flow, and he or she should be able
to compare the results in both legs.
Time-of-Flight (TOF) MRA
Initial reports used 2-dimensional
(2D) and 3-dimensional (3D) time-of-flight (TOF) MRA with limited
success. These methods rely on the detection of flow-related
phenomena to produce angiographic images. Images were degraded
by patient motion (primarily due to long scanning times, which
may been >1 h). Other causes of poor image quality include
turbulence, pulsating arteries, saturation, and poor signal-to-noise
ratios (SNRs). However, the TOF is still considered a better
technique than contrast-enhanced MRA for evaluating infrapopliteal
vessels because MRA depends on blood flow in the immediate vicinity
of the region imaged.
Contrast-enhanced 3D MRA has
become the method of choice. The technique relies on the detection
of contrast enhancement in the vascular lumen to produce findings
that are comparable to those of conventional catheter angiography
(Peripheralart-fig18; Peripheralart-fig19).
The current technique uses the bolus-chasing method material
in which vessels are imaged sequentially as contrast flows
distally.
Multiple overlapping fields of view are used, and images are
obtained in the coronal or sagittal planes (usually in 3 coronal
stations). This technique also uses subtraction to improve
the resultant vascular images by suppressing the background
and
reducing the volume averaging.
Images demonstrate the contrast-enhanced
anatomy of the arterial lumen. Stenosis is depicted as areas
of narrowing, and occlusion is depicted as areas of absent signal
intensity. Ulceration and aneurysm can also be defined.
Use of bolus-chasing MRA enables
radiologists to establish protocols for different studies by
adjusting the bolus dose and time, the infusion rate, the region
of interest, the section thickness, and the position in the
imaging plane by considering the purpose of the study, the patient's
condition, and the equipment available.
Bolus-chasing MRA is rapidly
evolving for many reasons such as the technology revolution
that made equipment widely available, improvements in technical
capabilities (eg, increased field strengths, dedicated coils,
increased SNRs, decreased repetition times, improved bolus-detection
techniques, MR SmartPrep technique). With these changes, along
with the increased familiarity and confidence of referring physicians
with this new modality, bolus-chasing MRA will replace conventional
catheter angiography.
False
Positives/Negatives: The
value of the study can be increased by using newer magnetic
resonance devices with higher resolution, by using gadolinium-based
contrast agent, and by having the expertise to interpret the
images. However, even when all available resources are used,
false-positive and false-negative results may still be encountered,
especially in patients who have undergone previous interventions.
For example, indwelling stents can cause severe artifacts and
may render findings inaccurate or nondiagnostic.
To date, no established data
are available; however, multiple studies reveal a sensitivity
and specificity of more than 90% with the bolus-chase technique.
The rate is slightly better in evaluating the iliac, femoral,
and popliteal segments.
Treatment
Treatment focuses on the relief
of symptoms and self-care to improve circulation.
Medications may be required to
control the disorder, including pain relievers, blood thinners,
and medications to enlarge or dilate the affected artery(ies).
Medications often are combined
with risk-factor modification and exercise to better relieve
symptoms and attempt to delay the progression of the disease.
Medications
Advice: aspirin every day, or
to take another platelet inhibitor such as clopidogrel (Plavix).
Medications, such as cilostazol (Pletal) and pentoxifylline
(Trental), also can help to decrease the symptoms of intermittent
claudication.
Surgery is usually performed
only on severe cases where the ability to work or pursue essential
activities is affected. Surgery may consist of removing the
lining of the artery (endarterectomy), or repairing or replacing
the vessel (grafting); most commonly, bypass surgery is performed,
using a vein or synthetic graft.
SURGERY
Surgery may be required to attempt
to treat clogged blood vessels.
Revascularization procedures
The goal of revascularization
is to improve circulation, either by opening narrowed arteries
or by bypassing the narrowed section of the artery using either
a section of vein taken from the leg or a synthetic graft.
These procedures, including
surgical and nonsurgical techniques, are used in people who
have severe or progressive symptoms, or whose leg pain occurs
at rest.
Before revascularization, the
location and extent of arterial narrowing is determined by MRI
or contrast angiography.
NONSURGICAL PROCEDURES
Balloon Angioplasty
The most common nonsurgical procedure
is percutaneous transluminal angioplasty, also called balloon
angioplasty (similar technique to that used to open the coronary
arteries, but performed on the blood vessels of the affected
extremity).This is usually done in the groin but can also be
done in the arm. In this procedure, a catheter is inserted into
the narrowed artery and a small balloon at the tip is briefly
inflated to open the narrowed vessel. This is often accompanied
by stent placement, in which a metallic implant is used as a
scaffold to widen and support the wall of the artery after it
is opened with the balloon.
The two most common types of
stents include the following:
Type 1 - Self-expandable stents
(eg, Wallstent, peripheralart -fig24)
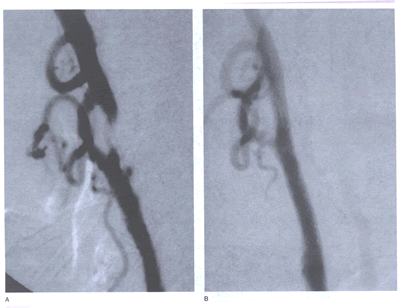
peripheralart-fig24.A. Carotid
angiogram of an asymptomatic, high-grade left internal carotid
artery stenosis. B. The lesion is corrected after treatment
with balloon angioplasty and the implantation of a Wallstent.
Type 2 - Balloon expandable stents
(eg, Palmaz stent) deployed by an angioplasty balloon
All of the published data show that best results are obtained
when the disease in common iliac artery, although the outcome
is also good when the disease is located in the superficial
femoral or popliteal artery.
The field of endovascular surgery
is growing rapidly, as are improvement in available instruments
and expertise. Currently, atherosclerotic iliac artery stenosis
responds well to simple balloon angioplasty, and it has the
best results of all of the peripheral vessels. Although many
complications and technical failures are still encountered,
the excellent results of endoluminal treatment in patients
with iliac artery occlusive disease and the relatively low
risk for
complications (compared with surgical revascularization) ensure
an enduring role for this modality. The application of this
study in other portions of the vascular tree is still being
investigated, but results are promising.
Emboli Protection Devices
The major cause of stroke during
carotid endarterectomy and percutaneous carotid intervention
is the procedural embolization of plaque debris along with platelet
and thrombin aggregates into the cerebral circulation. Transcranial
Doppler monitoring, a noninvasive method to detect echogenic
microemboli, has demonstrated frequent embolization during carotid
endarterectomy and stenting.
Although data are limited, there
appears to he a correlation between the number of emboli and
neurologic outcome after endarterectomy. Consequently, various
mechanical and pharmacologic approaches to prevent distal embolization
are currently under investigation to improve the safety of carotid
stenting.
Various mechanical devices to prevent embolization are under
investigation. Henry et al. reported their experience in 58
carotid artery stent procedures using a prototype cerebral protection
device and compared the results to 212 other patients treated
without the emboli protection device. This cerebral protection
catheter is a low-profile, balloon-tipped device designed to
block cerebral emboli when positioned in the internal carotid
artery distal to the target lesion. Conceptually, the protection
balloon occludes the run-off circulation to the brain, trapping
any particles dislodged following balloon angioplasty or stent
delivery so that they can subsequently be extracted via aspiration
into the guiding catheter. In this series, there was I immediate
neurologic complication (0.5 percent) compared to 1 I (5.2 percent)
in the group treated without the device. Feasibility of transient
carotid occlusion without consequences and potential endothelial
injury and embolization from the occlusion balloon itself, however,
are important concerns that need further evaluation.
An alternative mechanical embolization
device that allows continued perfusion while capturing emboli
has been developed (peripheralart-fig25 and 26 figs). This filter-type
device has been tested in carotid, coronary, and peripheral
interventions and should be available in the near future for
rigorous randomized trials.
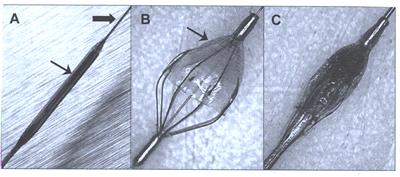
peripheralart-FIG 25 A. An emboli
protection device (Angioguard, Cordis) in closed state. The
bold arrow represents 0.014 in.
wire that leads the device (4 French)shown by the smaller arrow.
B. Open device with a filter with 100 µM pore size. C.
Closed filter with captured embolic debris.
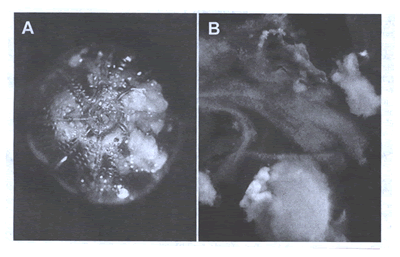
Peripheralart-fig26 A. Filter
with atheromatous embolic debris. B. Magnified view of typical
atheromatous embolic particles retrieved during intervention.
Angioplasty may require only
1 or 2 days in the hospital and may help the person avoid a
major operation. The procedure isn't painful but may be somewhat
uncomfortable because the person has to lie still on a hard
x-ray table. A mild sedative, but not general anesthesia, is
given. Afterward, the patient may be given heparin to prevent
blood clots from forming in the treated area. Many doctors prefer
giving patients a platelet inhibitor such as aspirin to prevent
clotting. A doctor can use ultrasound to check on the outcome
of the procedure and make sure that the narrowing doesn't recur.
Angioplasty can't be performed
if the narrowing is widespread, if it extends for a long distance,
or if the artery is severely and extensively hardened. Surgery
may be needed if a blood clot forms over the narrowed area,
a piece of the clot breaks off and blocks a more distant artery,
blood seeps into the lining of the artery causing it to bulge
and close off blood flow, or the person has bleeding (usually
from heparin given to prevent lotting).
Besides balloons, devices--including
lasers, mechanical cutters, ultrasonic catheters, stents, and
rotational sanders--are used to relieve obstructions. No one
device has proved superior.Endovascular procedures for lower
extremity PAD
Ballon Angoiplasty
Stenting.
When angioplasty does not restore
an adequate opening for blood to flow through the artery, a
stent can be used. A stent is an expandable wire-mesh tube that
can be deployed from a catheter at the location of the blockage.
The stent can provide ongoing mechanical support to open the
vessel and hold the plaque back. The stents currently available
are made from a variety of different metals.
Clinical trials with stents that
have different medications on them are being performed and have
great promise to increase the effectiveness of stents. Stenting
in leg arteries is similar to a coronary stenting.
Other catheter-based procedures.
Sometimes the material blocking
the artery is mainly clotted blood (a thrombus). In these
cases
there are a variety of catheters that use water jets to remove
the clot as he catheter is passed through it. This is called
thrombectomy.
Other catheters are made to deliver
drugs that speed the body’s natural ability to dissolve
clot. This is called thrombolysis.
There are still other catheters
that can remove plaque, called atherectomy catheters. Atherectomy
catheters are very rarely used for lower extremity PAD.
Catheter based procedures are often the first method of invasive
treatment used for patients with lower extremity PAD. This
can
usually be done on an outpatient basis and requires only some
local anesthetic. Patients can usually return to their normal
activity within one to two days. The downside of catheter-based
procedures is their relatively high recurrence rates. Most
severe
disease of the lower extremities extends over long segments
of the leg arteries and these long segments often develop
new
blockages after angioplasty or stenting. For that reason it
is important to examine the treated arteries with ultrasound
on a regular basis following the treatment so that any recurrence
can be detected earlyd treated easily before more extensive
surgery or other treatments are required.
Surgery
Surgery very often relieves symptoms, heals ulcers, and prevents
amputation. A vascular surgeon can sometimes remove a clot if
only a small area is blocked. Alternatively, a surgeon may put
in a bypass graft, in which a tube made of a synthetic material
or a vein from another part of the body is joined to the obstructed
artery above and below the obstruction. Another approach is
to remove the blocked or narrowed section and insert a graft
in its place. Cutting the nerves near the obstruction (an operation
called a sympathectomy) prevents the arteries from having spasms
and can be very helpful in some cases.
When amputation is needed to
cut out infected tissue, relieve unrelenting pain, or stop worsening
gangrene, surgeons remove as little of the leg as possible,
particularly if the person plans to wear an artificial limb.
Limbs with gangrene must be amputated
to prevent the death of the patient.
Self-care:
Exercise must be balanced with
rest. Walking or other activity, performed to the point of pain
and alternated with rest periods, is often recommended. Over
time, circulation improves because of the development of collateral
(new, small) blood vessels.
Stop smoking! Smoking constricts
arteries, decreases the blood's ability to carry oxygen and
increases the risk of forming clots (thrombi and emboli).
Foot care is particularly important
if diabetes is also present. Wear shoes that fit properly. Pay
attention to any cuts, scrapes or injury -- the tissues heal
slowly when there is decreased circulation and are prone to
infection.
If cholesterol is high, change
the diet to a low-cholesterol and low-fat diet.
Expectations (prognosis)
The prognosis depends on the
underlying disease and the stage at which peripheral vascular
disease is discovered. Removal of risk factors, such as smoking,
should be done immediately. In many cases, peripheral vascular
disease can be treated successfully but coexisting cardiovascular
problems may ultimately prove to be fatal.
In most people with peripheral
vascular disease, leg symptoms remain stable. About 10 percent
to 15 percent of patients improve, and about 15 percent to 20
percent worsen. Prognosis is better for people who are able
to remain tobacco-free, stay on a healthy diet, keep their blood
cholesterol under control, and exercise regularly
Complications
Injury or infection of the feet
and legs
Presence of open sores (ischemic ulcers) on the lower extremities
Ulcers on the feet and toes
Gangrene (tissue death) -- see gas gangrene
Arterial emboli
Impotence
Prevention
Control risk factors such as
obesity, high blood pressure, and smoking.
Treatment
If the person is a smoker, they should stop smoking immediately.
Exercise is essential to treating this disease. The patient
should walk until pain appears, rest until the pain disappears,
and then resume walking. The amount of walking a patient can
do should increase gradually as the symptoms improve. Ideally,
the patient should walk 30-60 minutes per day. Infections in
the affected area should be treated promptly.
Peripheral Arterial Disease Of
Abdominal Aorta and Its Major Branches
Occlusive arterial disease includes both coronary arteries,
which can lead to a heart attack, and peripheral arterial disease,
which may affect the abdominal aorta and its major branches
(abdominal aortic branches-fig2 and fig.vessels pad-fig3) as
well as the arteries of the legs as described above.
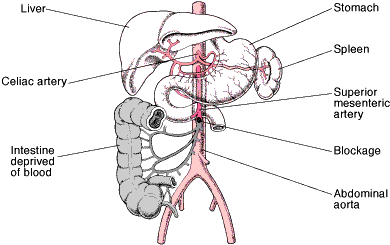
Fig.Abdominal aortic branches-fig2
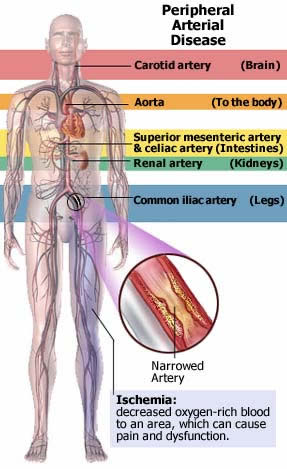
fig.vessels pad-fig3:Various
sites which may become involved in arteriosdclerosis.
Other peripheral arterial diseases
are Buerger's disease, Raynaud's disease, and acrocyanosis.
Most people with peripheral arterial
disease have atherosclerosis, a disease process in which fatty
material accumulates under the lining of the arterial wall,
gradually narrowing the artery. However, a partial or complete
occlusion of an artery can result from other causes, such as
a blood clot. When an artery narrows, the parts of the body
it serves may not receive enough blood. The resulting decrease
in oxygen supply (ischemia) can come on suddenly (acute ischemia)
or gradually (chronic ischemia).
To help prevent peripheral arterial
disease, a person should reduce the number of risk factors for
atherosclerosis, such as smoking, obesity, high blood pressure,
and high cholesterol levels.
Diabetes also is a major cause
of peripheral arterial disease, and appropriate treatment of
diabetes may delay the arterial disease.
Once peripheral arterial disease
appears, treatment is directed at its complications--severe
leg cramps while walking, angina, abnormal heart rhythms, heart
failure, heart attack, stroke, and kidney failure.
Abdominal Aorta and Branches
Obstruction of the abdominal
aorta and its major branches may be sudden or gradual. A sudden,
complete obstruction usually
results when a clot carried by the bloodstream lodges in
an artery (embolism), a clot forms (thrombosis) in a narrowed
artery,
or the artery wall tears (aortic dissection). An obstruction
that develops gradually usually results from atherosclerosis;
less often, it results from an abnormal growth of muscle
in
the artery wall or pressure from an expanding mass, such
as a tumor, outside the artery.
Symptoms
A sudden, complete obstruction
of the superior mesenteric artery, a major branch of the abdominal
aorta that supplies a large
part of the intestine, is an emergency. A person with such
an
obstruction becomes seriously ill and has severe abdominal
pain. Initially, vomiting and urgent bowel movements usually
occur.
Although the abdomen may feel tender when a doctor presses
on it, the severe abdominal pain is usually worse than the
tenderness,
which is widespread and vague. The abdomen may be slightly
distended. Through a stethoscope, a doctor initially hears
fewer bowel
sounds than normal in the abdomen. Later, no bowel sounds
can be heard. Blood appears in the stool, though at first it
can
be detected only by laboratory tests. Soon the stool looks
bloody.
Blood pressure falls, and the person goes into shock as the
intestine becomes gangrenous.
A gradual narrowing of the superior
mesenteric artery typically causes pain 30 to 60 minutes after
eating because digestion requires an increased blood flow to
the intestine. The pain is steady, severe, and usually centered
on the navel. This pain makes people afraid to eat, and they
may lose considerable weight. Because of the reduced blood supply,
nutrients may be poorly absorbed into the bloodstream, contributing
to the weight loss.
When a clot lodges in one of
the renal arteries, the branches that supply the kidneys, a
sudden pain occurs in the side, and the urine becomes bloody.
Gradual obstruction of the arteries to one or both kidneys usually
results from atherosclerosis and may lead to high blood pressure
(renovascular hypertension), which accounts for 5 percent of
all high blood pressure.
When the lower aorta is abruptly
obstructed where it divides into two branches that pass through
the pelvis to deliver blood to the legs (iliac arteries), both
legs suddenly become painful, pale, and cold. No pulse can be
felt in the legs, which may become numb.
When gradual narrowing occurs
in the lower aorta or one of the iliac arteries, the person
feels muscle tiredness or pain in the buttocks, hips, and calves
while walking. In men, impotence is common with narrowing of
the lower aorta or both iliac arteries. If the narrowing occurs
in the artery that begins at the groin and goes down the leg
to the knee (femoral artery), a person typically feels pain
in the calves while walking and has weak or no pulses below
the obstruction.
Treatment
Whether a person survives
a sudden obstruction of the superior mesenteric artery and
whether the intestine can be saved depend
on how fast the blood supply is restored. To save precious
time,
a doctor may send a patient for emergency surgery without
even taking x-rays. If the superior mesenteric artery is blocked
as the doctor suspects, only immediate surgery can restore
the
blood supply fast enough to save the person's life.
With a gradual obstruction of
blood flow to the intestine, nitroglycerin may relieve the abdominal
pain, but only surgery can relieve the obstruction. Doctors
use Doppler ultrasound and angiography to determine how extensive
the obstruction is and whether to operate.
Blood clots in the hepatic and
splenic arteries, the branches supplying the liver and spleen,
generally aren't as dangerous as obstructed blood flow to the
intestine. Even though an obstruction can cause injury to parts
of the liver or spleen, surgery is rarely needed to correct
the problem.
Early surgical removal of a clot
from a renal artery may restore kidney function. With a gradual
obstruction of a renal artery, doctors can sometimes use angioplasty
(a procedure in which a balloon is inserted into the artery
and inflated to clear the obstruction), but usually they must
remove or bypass the blockage surgically.
Emergency surgery can clear a
sudden obstruction of the lower aorta where it divides into
two branches to deliver blood to the legs. Sometimes doctors
can dissolve the clot by injecting a thrombolytic drug, such
as urokinase, but surgery is more likely to be successful.
Arteries of the Legs and Arms(see
above)
With a gradually narrowing leg artery, the first symptom is
a painful, aching, cramping, or tired feeling in leg muscles
during physical activity; this feeling is called intermittent
claudication. Muscles hurt when the person walks, and the pain
comes on faster and is more severe when the person walks quickly
or uphill. Most commonly, the pain is in the calf, but it can
also be in the foot, thigh, hip, or buttocks, depending on the
location of the narrowing. The pain can be relieved by resting.
Usually, after 1 to 5 minutes of sitting or standing, the person
can walk the same distance already covered before feeling pain
again. The same kind of pain on exertion is also caused by narrowing
of the arteries in the arms.
As the disease gets worse, the
distance the person can walk without pain gets shorter. Eventually,
the muscles may ache even at rest. The pain usually begins in
the lower leg or foot, is severe and unrelenting, and gets worse
when the leg is elevated. The pain often prevents sleep. For
relief, a person may hang the feet over the side of the bed
or rest sitting up with the legs hanging down.
A foot with a severely reduced
blood supply is usually cold and numb. The skin may be dry and
scaly, and the nails and hair may not grow well. As the obstruction
worsens, a person may develop sores, typically on the toes or
heel and occasionally on the lower leg, especially after an
injury. The leg may shrink. A severe obstruction may cause tissue
death (gangrene).
With a sudden, complete obstruction
of a leg or arm artery, a person feels severe pain, coldness,
and numbness. The person's leg or arm is either pale or bluish
(cyanotic). No pulse can be felt below the obstruction.
Diagnosis:see above
Treatment
People with intermittent claudication should walk at least 30
minutes a day, if possible. When they feel pain, they should
stop until it subsides and then walk again. By doing this, they
can usually increase the distance they can walk comfortably,
probably because the exercise improves muscle function and makes
other blood vessels supplying the muscles grow larger. People
with obstructions shouldn't use tobacco in any form. Elevating
the head of the bed with 4- to 6-inch blocks may help by increasing
blood flow to the legs.
Doctors may prescribe a drug
such as pentoxifylline in an effort to improve oxygen delivery
to the muscles. Other drugs, such as calcium antagonists or
aspirin, also may be helpful. Beta-blockers, which help people
with coronary artery obstructions by slowing the heart and reducing
its need for oxygen, sometimes worsen symptoms in people with
leg artery obstructions.
Performing Foot Care
A person with poor circulation to the feet should use these
self-care measures and precautions:
Inspect feet daily for cracks,
sores, corns, and calluses.
Wash feet daily in lukewarm water with mild soap and dry them
gently and thoroughly.
Use a lubricant, such as lanolin, for dry skin.
Use unmedicated powder to keep the feet dry.
Cut toenails straight across and not too short. (A podiatrist
may have to cut the nails.)
Have a podiatrist treat corns or calluses.
Don't use adhesive or harsh chemicals.
Change socks or stockings daily and shoes often.
Don't wear tight garters or stockings with tight elastic tops.
Wear loose wool socks to keep the feet warm.
Don't use hot water bottles or heating pads.
Wear shoes that fit well and have wide toe spaces.
Ask the podiatrist about a prescription for special shoes if
the foot is deformed.
Don't wear open shoes or walk barefoot.
Foot Care
The goal of foot care is to protect circulation to the foot
and prevent complications of poor circulation. A person with
foot ulcers requires meticulous care to prevent further deterioration
that would make amputation of the foot necessary. The ulcer
must be kept clean: It should be washed daily with mild soap
or salt solution and covered with clean, dry dressings. A person
with a foot ulcer may need complete bed rest with the head of
the bed raised. A person who has diabetes also must control
blood sugar levels as well as possible. As a rule, anyone with
poor circulation to the feet or with diabetes should have a
doctor check any foot ulcer that isn't healing after about 7
days. Many times, a doctor prescribes an antibiotic ointment.
If the ulcer becomes infected, the doctor generally prescribes
antibiotics to be taken by mouth. Healing may take weeks or
even months.
Buerger's Disease
Buerger's disease (thromboangiitis obliterans) is the obstruction
of small and medium-sized arteries and veins by inflammation
triggered by smoking.
Men ages 20 to 40 who smoke cigarettes
get Buerger's disease more than anyone else. Only about 5 percent
of people with the disease are women. Although no one knows
what causes Buerger's disease, only smokers get it, and continuing
to smoke makes it worse. Because only a small number of smokers
get Buerger's disease, some people must be more susceptible
than others. Why and how cigarette smoke causes the problem
aren't known.
Symptoms
Symptoms of reduced blood supply to the arms or legs develop
gradually, starting at the fingertips or toes and progressing
up the arms or legs, eventually causing gangrene. About 40 percent
of people with this disease also have episodes of inflammation
in the veins, particularly the superficial veins, and the arteries
of the feet or legs. People may feel coldness, numbness, tingling,
or burning before their doctor sees any signs. They often have
Raynaud's phenomenon (see page 136 in this chapter) and get
muscle cramps, usually in the arches of their feet or in their
legs but rarely in their hands, arms, or thighs. With more severe
obstruction, the pain is worse and lasts longer. Early in the
disease, ulcers, gangrene, or both may appear. The hand or foot
feels cold, sweats a lot, and turns bluish, probably because
the nerves are reacting to severe, persistent pain.
Diagnosis
In more than 50 percent of people with Buerger's disease, the
pulse is weak or absent in one or more arteries of the feet
or wrists. Often, the affected hands, feet, fingers, or toes
become pale when raised above the heart and red when lowered.
People may develop skin ulcers and gangrene, usually of one
or more fingers or toes.
Ultrasound tests reveal a severe
decrease in blood pressure and blood flow in the affected feet,
toes, hands, and fingers. Angiograms (x-rays of the arteries)
show obstructed arteries and other circulation abnormalities,
especially in the hands and feet.
Treatment
A person with this disease must stop smoking, or it will relentlessly
worsen, and ultimately an amputation may be necessary. Also,
the person should avoid exposure to the cold; injuries from
heat, cold, or substances such as iodine or acids used to treat
corns and calluses; injuries from poorly fitting shoes or minor
surgery (such as trimming calluses); fungal infections; and
drugs that can narrow blood vessels.
Walking 15 to 30 minutes twice
a day is recommended, except for people with gangrene, sores,
or pain at rest; they may need bed rest. People should protect
their feet with bandages that have heel pads or with foam-rubber
booties. The head of the bed can be raised on 6- to 8-inch blocks
so gravity helps blood flow through the arteries. Doctors may
prescribe pentoxifylline, calcium antagonists, or platelet inhibitors
such as aspirin, especially when the obstruction results from
spasm.
For people who quit smoking but
still have arterial occlusion, surgeons may improve blood flow
by cutting certain nearby nerves to prevent spasm. They seldom
perform bypass grafts, because the arteries affected by this
disease are too small.
Functional Peripheral Arterial
Disorders
Most of these disorders result from a spasm of arteries in the
arms or legs. They may be caused by a fault in the blood vessels
or by disturbances in the nerves that control the widening and
narrowing of arteries (sympathetic nervous system). Such nerve
defects may themselves be a consequence of blockage from atherosclerosis.
Raynaud's Disease and Raynaud's
Phenomenon
Raynaud's disease and Raynaud's phenomenon are conditions in
which small arteries (arterioles), usually in the fingers and
toes, go into spasm, causing the skin to become pale or a patchy
red to blue.
Doctors use the term Raynaud's
disease when no underlying cause is apparent and the term Raynaud's
phenomenon when a cause is known. Sometimes, the underlying
cause can't be diagnosed at first, but usually it becomes apparent
within 2 years.
Between 60 and 90 percent of
the cases of Raynaud's disease occur in young women.
Causes
Possible causes include scleroderma,
rheumatoid arthritis, atherosclerosis, nerve disorders, decreased
thyroid activity,
injury, and reactions
to certain drugs, such as ergot and methysergide. Some people
with Raynaud's phenomenon also have migraine headaches, variant
angina, and high blood pressure in their lungs (pulmonary
hypertension). These associations suggest that the cause of
the arterial spasms
may be the same in all these disorders. Anything that stimulates
the sympathetic nervous system, such as emotion or exposure
to cold, can cause arterial spasms.
Symptoms and Diagnosis
Spasm of small arteries in
the fingers and toes comes on quickly, most often triggered
by exposure to cold. It may last minutes
or hours. The fingers and toes turn white, usually in a spotty
fashion. Only one finger or toe or parts of one or more may
be affected, turning a patchy red and white. As the episode
ends, the affected areas may be pinker than usual or bluish.
The fingers or toes usually don't hurt, but numbness, tingling,
a pins-and-needles sensation, and a burning sensation are
common. Rewarming the hands or feet restores normal color and
sensation.
However, when people have long-standing Raynaud's phenomenon
(especially those with scleroderma), the skin of the fingers
or toes may change permanently--appearing smooth, shiny,
and tight. Small painful sores may appear on the tips of the
fingers
or toes.
To distinguish between arterial
blockage and arterial spasm, doctors perform laboratory tests
before and after someone is exposed to cold.
Treatment
People can control mild Raynaud's disease by protecting their
trunk, arms, and legs from cold and by taking mild sedatives.
They must stop smoking because nicotine constricts blood vessels.
For a few people, relaxation techniques, such as biofeedback,
may reduce the spasms. Raynaud's disease is commonly treated
with prazosin or nifedipine. Phenoxybenzamine, methyldopa, or
pentoxifylline occasionally helps. When people have progressive
disability and other treatment doesn't work, sympathetic nerves
may be cut to relieve the symptoms, but the relief may last
only 1 to 2 years. This operation, called a sympathectomy, generally
is more effective for people with Raynaud's disease than for
those with Raynaud's phenomenon.
Doctors treat Raynaud's phenomenon
by treating the underlying disorder. Phenoxybenzamine may help.
Drugs that may constrict blood vessels (such as beta-blockers,
clonidine, and ergot preparations) may make Raynaud's phenomenon
worse.
Acrocyanosis
Acrocyanosis is a persistent, painless blueness of both hands
and, less commonly, the feet, caused by unexplained spasm of
the small blood vessels of the skin.
The disorder usually occurs in
women, not necessarily those with occlusive arterial disease.
The fingers or toes and hands or feet are constantly cold and
bluish and sweat profusely; they may swell. Cold temperatures
usually intensify the blue coloring, and warming reduces it.
The condition isn't painful and doesn't damage the skin.
Doctors diagnose the disorder
based on persistent symptoms limited to the person's hands and
feet along with normal pulses. Treatment is usually unnecessary.
Doctors may prescribe drugs that dilate the arteries, but they
usually don't help. Very rarely, sympathetic nerves are cut
to relieve symptoms.
REFLEX SYMPATHETHIC DYSTROPHY
SYNDROME
Reflex sympathetic dystrophy
syndrome (RSDS) is a chronic condition characterized by severe
burning pain, pathological changes in bone and skin,excessive
sweating, tissue swelling, and extreme sensitivity to touch.
The syndrome is a nerve disorder
that occurs at the site of an injury (most often to the arms
or legs). It occurs especially after injuries from high-velocity
impacts such as those from bullets or shrapnel. However, it
may occur without apparent injury.
One visible sign of RSDS near
the site of injury is warm, shiny red skin that later becomes
cool and bluish.The pain that patients report is out of proportion
to the severity of the injury and gets worse, rather than better,
over time.
Eventually the joints become
stiff from disuse, and the skin, muscles, and bone atrophy.
The symptoms of RSDS vary in severity and duration.
The cause of RSDS is unknown.
The disorder is unique in that it simultaneously affects the
nerves, skin, muscles, blood vessels, and bones.
RSDS can strike at any age but
is more common between the ages of 40 and 60, although the number
of RSDS cases among adolescents and young adults is increasing.
RSDS is diagnosed primarily through
observation of the symptoms. Some physicians use thermography
to detect changes in body temperature that are common in RSDS.
X-rays may also show changes
in the bone.
Is there any treatment?
Physicians use a variety of drugs to treat RSDS. Elevation of
the extremity and physical therapy are also used to treat RSDS.
Injection of a local anestheticis usually the first step in
treatment. TENS (transcutaneous electrical stimulation), a procedure
in which brief pulses of electricity are applied to nerve endings
under the skin, has helped some patients in relieving chronic
pain. In some cases, surgical or chemical sympathectomy -- interruption
of the affected portion of the sympathetic nervous system --
is necessary to relieve pain. Surgical sympathectomy involves
cutting the nerve or nerves, destroying the pain almost instantly,
but surgery may also destroy other sensations as well.
What is the prognosis?
Good progress can be made in treating RSDS if treatment is begun
early, ideally within three months of the first symptoms. Early
treatment often results in remission. If treatment is delayed,
however, the disorder can quickly spread to the entire limb,
and changes in bone and muscle may become irreversible. In 50
percent of RSDS cases, pain persists longer than 6months and
sometimes for years.
What is reflex sympathetic dystrophy/complex
regional pain syndrome?
RSD/CRPS is a chronic condition
characterized by severe burning pain, pathological changes in
bone and skin, excessive sweating, tissue swelling, and extreme
sensitivity to touch. The syndrome is a nerve disorder that
occurs at the site of an injury (most often to the arms or legs).
It occurs especially after injuries from high-velocity impacts
such as those from bullets or shrapnel. However, it may occur
without apparent injury.
The condition called "causalgia"
was first documented in the 19th century by physicians concerned
about pain that Civil War veterans continued to experience after
their wounds had healed. Doctors often called it "hot pain,"
after its primary symptom. Over the years, the syndrome was
classified as one of the peripheral neuropathies, and later,
as a chronic pain syndrome. Currently, there are two types of
CRPS that are differentiated-type I and type II. Both types
share the same basic set of symptoms, but have one distinct
difference: type I (previously referred to as RSD) describes
cases in which there is no nerve injury, while type II (formerly
called causalgia) refers to cases in which a distinct nerve
injury, for example from a gunshot wound, has occurred
What are the symptoms of RSD/CRPS?
The symptoms of RSD/CRPS usually occur near the site of an injury,
either major or minor, and include: burning pain, muscle spasms,
local swelling, increased sweating, softening of bones, joint
tenderness or stiffness, restricted or painful movement, and
changes in the nails and skin. One visible sign of RSD/CRPS
near the site of injury is warm, shiny red skin that later becomes
cool and bluish.
The pain that patients report
is out of proportion to the severity of the injury and gets
worse, rather than better, over time. It is frequently characterized
as a burning, aching, searing pain, which may initially be localized
to the site of injury or the area covered by an injured nerve
but spreads over time, often involving an entire limb. It can
sometimes even involve the opposite extremity. Pain is continuous
and may be heightened by emotional stress. Moving or touching
the limb is often intolerable. Eventually the joints become
stiff from disuse, and the skin, muscles, and bone atrophy.
The symptoms of RSD/CRPS vary
in severity and duration. However, there are usually three stages
associated with RSD/CRPS, and each stage is marked by progressive
changes in the skin, nails, muscles, joints, ligaments, and
bones. Stage one lasts from 1 to 3 months and is characterized
by severe, burning pain at the site of the injury. Muscle spasm,
joint stiffness, restricted mobility, rapid hair and nail growth,
and vasospasm (a constriction of the blood vessels) that affects
color and temperature of the skin can also occur.
In stage two, which lasts from
3 to 6 months, the pain intensifies. Swelling spreads, hair
growth diminishes, nails become cracked, brittle, grooved, and
spotty, osteoporosis becomes severe and diffuse, joints thicken,
and muscles atrophy.
As the patient reaches stage
three, changes in the skin and bones become irreversible, and
pain becomes unyielding and may now involve the entire limb.
There is marked muscle atrophy, severely limited mobility of
the affected area, and flexor tendon contractions (contractions
of the muscles and tendons that flex the joints). Occasionally
the limb is displaced from its normal position, and marked bone
softening is more dispersed.
What causes RSD/CRPS?
RSD/CRPS was originally thought to be the result of malfunctioning
nerves of the sympathetic nervous system-the part of the nervous
system responsible, for example, for controlling the diameter
of blood vessels. This idea has been called into question and
the mechanism remains controversial.
Since RSD/CRPS is most often
caused by trauma to the extremities, other conditions that can
bring about RSD/CRPS include sprains, fractures, surgery, damage
to blood vessels or nerves, and cerebral lesions. The disorder
is unique in that it simultaneously affects the nerves, skin,
muscles, blood vessels, and bones.
Who gets it?
RSD/CRPS can strike at any age, but has usually been more common
between the ages of 40 and 60. Recent reports show that the
number of RSD/CRPS cases among adolescents and young adults
is increasing. It affects both men and women, but is most frequently
seen in women.
Investigators estimate that two
to five percent of those with peripheral nerve injury and 12
to 21 percent of those with hemiplegia (paralysis of one side
of the body) will suffer from RSD/CRPS.
How is RSD/CRPS diagnosed?
RSD/CRPS is often misdiagnosed because it remains poorly understood.
Diagnosis is complicated by the fact that some patients improve
without treatment. A delay in diagnosis and/or treatment for
this syndrome can result in severe physical and psychological
problems. Early recognition and prompt treatment provide the
greatest opportunity for recovery.
RSD/CRPS is diagnosed primarily
through observation of the symptoms. However, some physicians
use thermography — a diagnostic technique for measuring
blood flow by determining the variations in heat emitted from
the body — to detect changes in body temperature that are
common in RSD/CRPS. A color-coded "thermogram" of
a person in pain often shows an altered blood supply to the
painful area, appearing as a different shade (abnormally pale
or violet) than the surrounding areas of the corresponding part
on the other side of the body. An abnormal thermogram in a patient
who complains of pain may lead to a diagnosis of RSD/CRPS. X-rays
may also show changes in the bone.
What is the prognosis?
Good progress can be made in treating RSD/CRPS if treatment
is begun early, ideally within 3 months of the first symptoms.
Early treatment often results in remission. If treatment is
delayed, however, the disorder can quickly spread to the entire
limb and changes in bone and muscle may become irreversible.
In 50 percent of RSD/CRPS cases, pain persists longer than 6
months and sometimes for years.
What is the treatment?
Physical therapy is the mainstay of therapy. Physicians use
a variety of drugs to treat RSD/CRPS, including corticosteroids,
vasodilators, and alpha- or beta-adrenergic-blocking compounds.
Elevation of the extremity may be helpful. Injection of a local
anesthetic, such as lidocaine, is sometimes used. Injections
are repeated as needed. TENS (transcutaneous electrical stimulation),
a procedure in which brief pulses of electricity are applied
to nerve endings under the skin, has helped some patients in
relieving chronic pain.
In some cases, surgical or chemical
sympathectomy-interruption of the affected portion of the sympathetic
nervous system-has been used to relieve pain. Surgical sympathectomy
involves cutting the nerve or nerves, destroying the pain almost
instantly. But surgery is controversial and may also destroy
other sensations.
Are there any other disorders
like RSDPS?/CR
RSD/CRPS has characteristics similar to those of other disorders,
such as shoulder-hand syndrome, which sometimes occurs after
a heart attack and is marked by pain and stiffness in the arm
and shoulder; Sudeck's syndrome, which is prevalent in older
people and in women and is characterized by bone changes and
muscular atrophy, but is not always associated with trauma;
and Steinbrocker's syndrome, which affects both sexes but is
slightly more prevalent in women, and includes such symptoms
as gradual stiffness, discomfort, and weakness in the shoulder
and hand.
What research is being done?
The National Institute of Neurological Disorders and Stroke
(NINDS), a part of the National Institutes of Health (NIH),
supports and conducts research on the brain and central nervous
system. Some studies are conducted at the Institute's own laboratories
and clinics located in Bethesda, Maryland, on the NIH campus,
while others are funded through grants to major medical institutions
across the country. NINDS-supported scientists are studying
new approaches to treat RSD/CRPS and intervene more aggressively
after traumatic injury to lower the patient's chances of developing
the disorder. Other studies to overcome chronic pain syndromes
are discussed in the pamphlet " Pain: Hope Through Research,"
published by the NINDS.
Is help available?
The unrelenting pain from RSD/CRPS has caused many patients
much physical and emotional misery. Family, friends, coworkers,
and, regrettably, physicians themselves, may regard the patient
as a complainer, thereby increasing the patient's distress.
To meet the needs of individuals with RSD/CRPS and other conditions
causing chronic pain, the following voluntary health agencies
promote research, provide information, and may offer advice
on coping. For information, write or call:
Reflex Sympathetic Dystrophy
Syndrome Association (RSDSA)
P.O. Box 502
Milford, CT 06460
info@rsds.org
http://www.rsds.org
Tel: 203-877-3790
Fax: 203-882-8362
American RSDHope Group
P.O. Box 875
Harrison, ME 04040-0875
rsdhope@mail.org
http://www.rsdhope.org/
Tel: 207-583-4589
American Chronic Pain Association
(ACPA)
P.O. Box 850
Rocklin, CA 95677-0850
ACPA@pacbell.net
http://www.theacpa.org
Tel: 916-632-0922 800-533-3231
Fax: 916-632-3208
National Chronic Pain Outreach
Association (NCPOA)
P.O. Box 274
Millboro, VA 24460
ncpoa@cfw.com
http://www.chronicpain.org
Tel: 540-862-9437
Fax: 540-862-9485
Mayday Fund [For Pain Research]
c/o SPG
136 West 21st Street, 6th Floor
New York, NY 10011
MaydyFnd@aol.com
http://www.painandhealth.org
Tel: 212-366-6970
Fax: 212-838-2896
For information on other neurological
disorders or research programs funded by the National Institute
of Neurological Disorders and Stroke, contact the Institute's
Brain Resources and Information Network (BRAIN) at:
BRAIN
P.O. Box 5801
Bethesda, Maryland 20824
301-496-5751
800-352-9424
www.ninds.nih.gov
Reviewed April 21, 2003